 III.
DNA, RNA, and Chromosome Structure
III.
DNA, RNA, and Chromosome Structure
 III.
DNA, RNA, and Chromosome Structure
III.
DNA, RNA, and Chromosome StructureV. Origin of Life Hypotheses
VI. Photosynthesis
VII. Cellular Respiration
 We
have already described one way that some organisms harvest energy from the environment:
they transform radiant energy into chemical energy. Of course, only photosynthetic
organisms perform those reactions. Now, we will examine the energy harvesting
reactions that ALL living cells perform: Cellular Respiration. All
living cells - eubacteria, archaea, protists, fungi, plants, and animals - can
harvest the energy contained in the chemical bonds of complex organic molecules.
By breaking the covalent bonds between carbon atoms in these molecules, energy
is released. The energy released by these catabolic reactions is used to bind
ADP + P --> ATP in coupled, anabolic reactions. As such, some of the energy
in the covalent bonds of the initial organic molecules is transformed into chemical
energy in bonds of ATP. Energy in this form is now available to all of the enzymes
in the cell, for catalyzing their own reactions (chemical energy) or doing work
like muscular contraction (mechanical energy) or pumping ions across a membrane
against their concentration gradient (active transport).
We
have already described one way that some organisms harvest energy from the environment:
they transform radiant energy into chemical energy. Of course, only photosynthetic
organisms perform those reactions. Now, we will examine the energy harvesting
reactions that ALL living cells perform: Cellular Respiration. All
living cells - eubacteria, archaea, protists, fungi, plants, and animals - can
harvest the energy contained in the chemical bonds of complex organic molecules.
By breaking the covalent bonds between carbon atoms in these molecules, energy
is released. The energy released by these catabolic reactions is used to bind
ADP + P --> ATP in coupled, anabolic reactions. As such, some of the energy
in the covalent bonds of the initial organic molecules is transformed into chemical
energy in bonds of ATP. Energy in this form is now available to all of the enzymes
in the cell, for catalyzing their own reactions (chemical energy) or doing work
like muscular contraction (mechanical energy) or pumping ions across a membrane
against their concentration gradient (active transport).
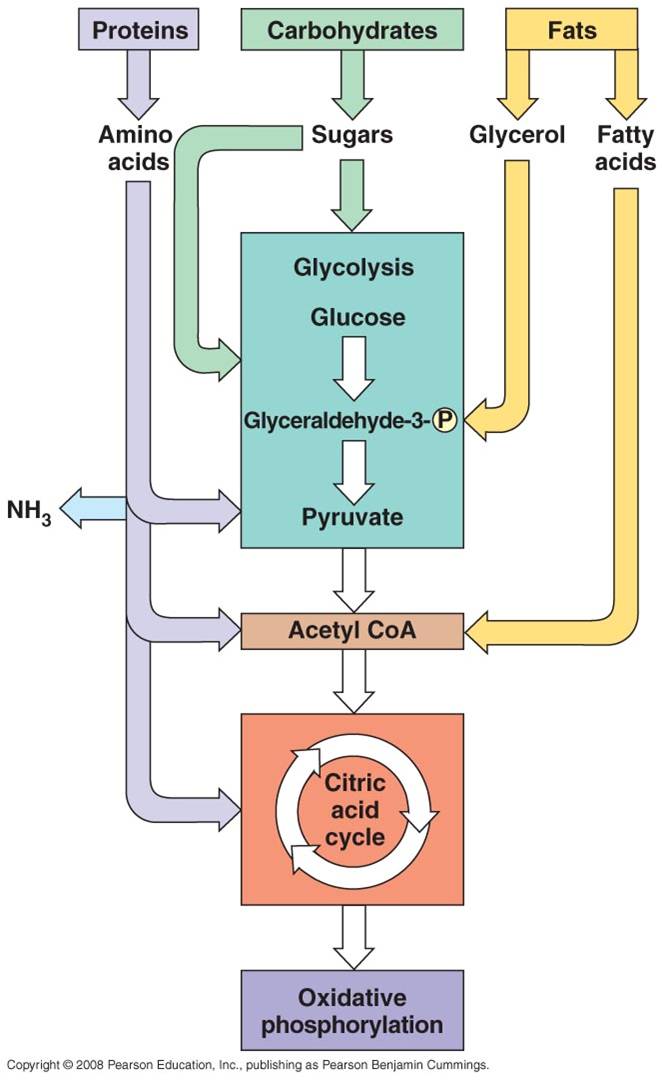
All four classes of biological molecules (carbo's, fats, proteins, and nucleic acids) are broken down for energy harvest. The process of carbohydrate metabolism, however, is the central process. Fats, proteins, and nucleic acids are broken into their monomers, these are modified, and then these products can be shunted into the carbohydrate digestion process. So, although we will focus on carbohydrate metabolism - and glucose metabolism in particular - you should appreciate that all other polymers can be broken down for energy harvest. And respiration not only harvests energy - respiration also provides the monomers needed by the cell to build its own biomolecules. So, when you digest protein, energy is harvested and the separated amino acids can be used by your cells to make your DNA-specified proteins. This is why a balanced diet is important - digestion of varied complex organic molecules provides the different monomers and other essential vitamins and minerals (often used as cofactors in reactions) that your cells require.
The metabolism of glucose can accur in the presence of absence of oxygen. The first step is glycolysis, in which the six-carbon sugar is split into 2 C3 molecules of pyruvate. The breaking of this bond releases a small amount of energy. In the absence of oxygen, fermentation occurs. The primary function of this "anaerobic" respiration is to recyclce some chemicals needed to keep glycolysis going. So, anaerobic respiration, including glycolysis and fermentation, breaks only a couple bonds and produces only a small amount of energy. In the presence of oxygen, the pyruvates can be completely oxidized. The C3 molecules are completely broken down into 3 one-carbon molecules of carbon dioxide. The complete breakdown of the the pyruvates releases much more energy. This is probably why aerobic organisms have come to dominate the planet - they harvest more energy from the food they consume, and can use this energy to survive and reproduce more effectively.
1. Glycolysis
a. the process:
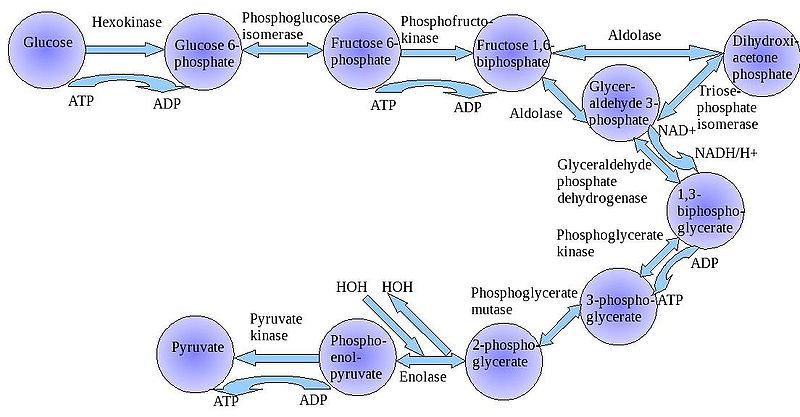 The
"splitting of glucose" (glyco-lysis) is probably an ancient metabolic
reaction; it is performed in the cytoplasm of ALL living cells from prokaryotes
to eukaryotes, and cells can perform this reaction in the presence OR absence
of oxygen gas. So, it seems likely that this was an important energy harvesting
reaction for ancient cells that lived before ~2 bya - before oxygen became abundant
in the oceans and atmosphere. As you can see in the flowchart, glycolysis is
not ONE reaction - it is a series of reactions catalyzed by a variety of enzymes.
For our purposes here, we will consider the primary "inputs" and "outputs"
of the entire reaction, rather than concerning ourselves with each step.
The
"splitting of glucose" (glyco-lysis) is probably an ancient metabolic
reaction; it is performed in the cytoplasm of ALL living cells from prokaryotes
to eukaryotes, and cells can perform this reaction in the presence OR absence
of oxygen gas. So, it seems likely that this was an important energy harvesting
reaction for ancient cells that lived before ~2 bya - before oxygen became abundant
in the oceans and atmosphere. As you can see in the flowchart, glycolysis is
not ONE reaction - it is a series of reactions catalyzed by a variety of enzymes.
For our purposes here, we will consider the primary "inputs" and "outputs"
of the entire reaction, rather than concerning ourselves with each step.
Through this series of reactions, the six-carbon glucose is modified and split into 2 C3 molecules of pyruvate. Glycolysis requires an input of energy to "get the reaction going". This activation energy is provided by 2 ATP. A phosphate is transferred from each ATP to the terminal carbons on the glucose. These phosphates destabilize the glucose, and also give it a charge - it will not diffuse back across the lipid bilayer. The splitting of the molecule releases energy and high energy electrons. Some of the energy is used to phosphorylate 4 ADP--> 4 ATP. Thus, although 2 ATP were used to start the reaction, there is a net gain of 2 ATP. The high energy electrons are accepted by an important molecule called NAD (nicotinamide adenine dinucleotide). With the acceptance of an electron, each NAD becomes negative charged (NAD-) and reacts with a H+ ion in solution - making NADH. So, NAD is a low energy form of the molecule, and NADH is a high energy form of the molecule.
So, for our purposes here, we can summarize glycolysis as:
glucose (C6) + 2 ATP + 2NAD ----> 2 pyruvate (C3) + 4 ATP + 2NADH
b. Requirements:
In order for glycolysis to occur (and all subsequent glucose metabolism!!!) the cell must have all three reactants - Glucose, ATP, and NAD. Obviously, if glucose is absent then the cell starves. That's moot. And, As glycolysis proceeds, there is always a net surplus of ATP produced by previous glycolysis reactions. But what about NAD? As glycolysis proceeds, extra ATP is produced that can be used in subsequent reactions to keep metabolism going. However, NAD is used up and converted to NADH. If NAD is not present, glycolysis stops (very BAD).
c. Solutions:
So, NADH must give up its electrons (and H+) to something else, so that the NAD can be recycled and used in glycolysis. This happens two ways, depending on whether oxygen is present (aerobic respiration) or absent (anearobic respiration). In the absence of oxygen, the NADH passed the electron back to pyruvate, and either lactate or ethanol is formed. No new energy is released from these reactions, so the energy produced by anaerobic respiration is just what is produced in glycolysis - fermentation is just a mechanism for the cell to recycle the NAD to keep glycolysis going. In the presence of oxygen, the pyruvates can be metabolized more completely - splitting all the carbon-carbon bonds and releaseing much more energy. Indeed, this is the primary benefit of aerobic respiration; but the recycling of NAD also happens during the process.
2. Anaerobic Respiration
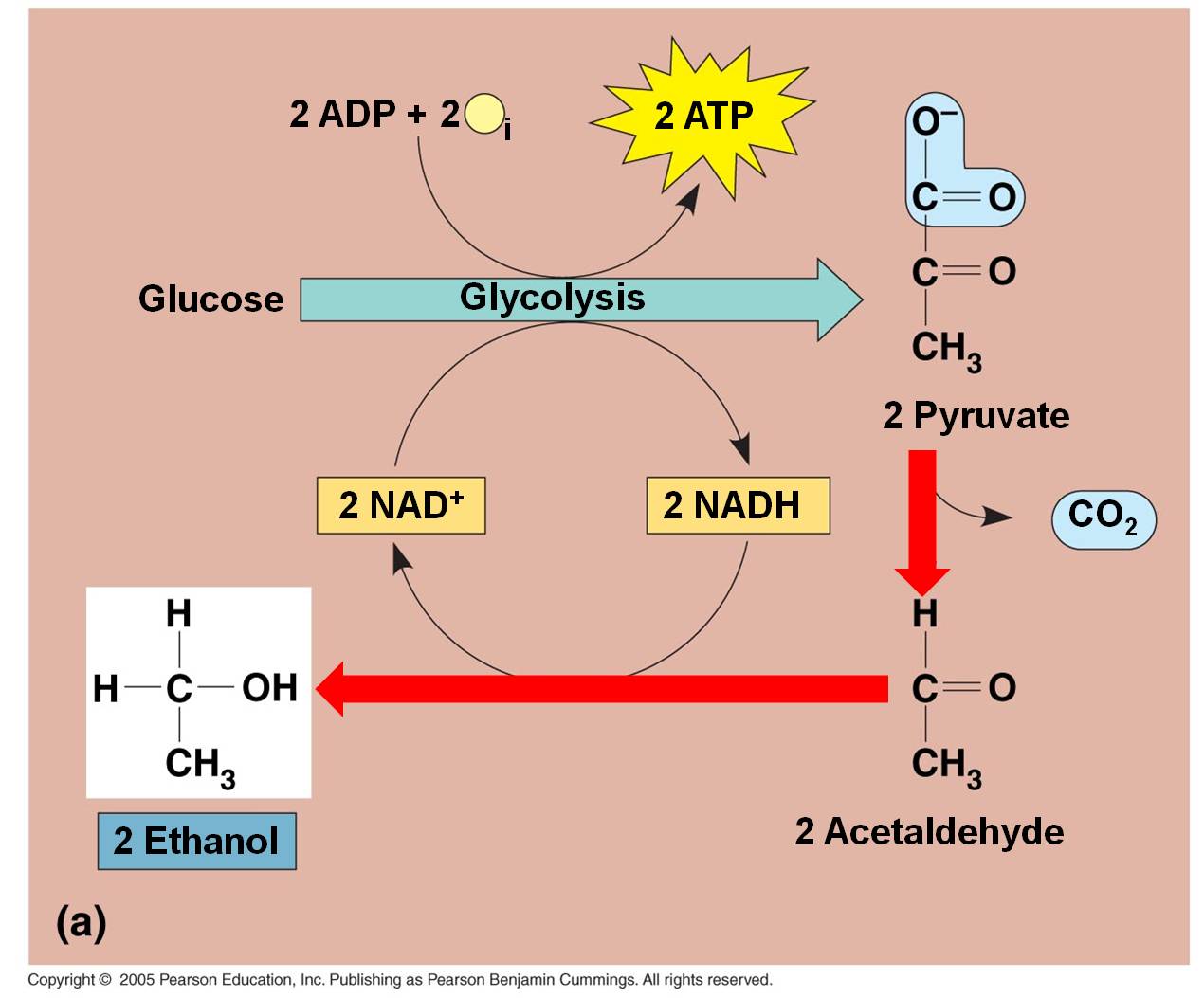 These
pathways take place in the absence of oxygen. For aerobic organisms that need
oxygen to survive (eukaryotes and most prokaryotes) these are temporary, facultative
reactions meant to "weather the storm" while oxygen concentrations
are low. If oxygen concentrations remain low, eukaryotic cells will not be able
to survive on these reactions, alone, and they will die. Because they are smaller
and have lower energy demands, some facultatively anaerbic bacteria can sustain
themselves on these reactions. Some organisms are obligate anaerobes (some eubacteria
and most archaea) that use these pathways all the time and are poisoned by oxygen.
Indeed, some bacteria perform both these types of fermentation reactions at
once, with one pyruvate converted to lactic acid while the other is metabolized
to alcohol.
These
pathways take place in the absence of oxygen. For aerobic organisms that need
oxygen to survive (eukaryotes and most prokaryotes) these are temporary, facultative
reactions meant to "weather the storm" while oxygen concentrations
are low. If oxygen concentrations remain low, eukaryotic cells will not be able
to survive on these reactions, alone, and they will die. Because they are smaller
and have lower energy demands, some facultatively anaerbic bacteria can sustain
themselves on these reactions. Some organisms are obligate anaerobes (some eubacteria
and most archaea) that use these pathways all the time and are poisoned by oxygen.
Indeed, some bacteria perform both these types of fermentation reactions at
once, with one pyruvate converted to lactic acid while the other is metabolized
to alcohol.
a. In plants, fungi, and bacteria:
In the absence of oxygen, the C3 pyruvates are broken into a C2 molecule and CO2. The NADH releases its electron and hydrogen to the C2 molecule, forming ETHANOL (alcohol). The NAD can be recycled and glycolysis can continue, even in the absence of oxygen. This is alcohol fermentation. Of course, the only energy produced by the "glycolysis and fermentation" pathway is a net of 2 ATP per glucose. That is not alot of energy. In a confined environment (like a beer keg or a wine cask), the alcohol concentration may rise to levels that are toxic to the fermenting yeasts. Different yeasts have different tolerances, but even the most tolerant die at about 20% alcohol concentrations. Humans increase the alcohol concentration of fluids by 'distilling' the products of fermentation.
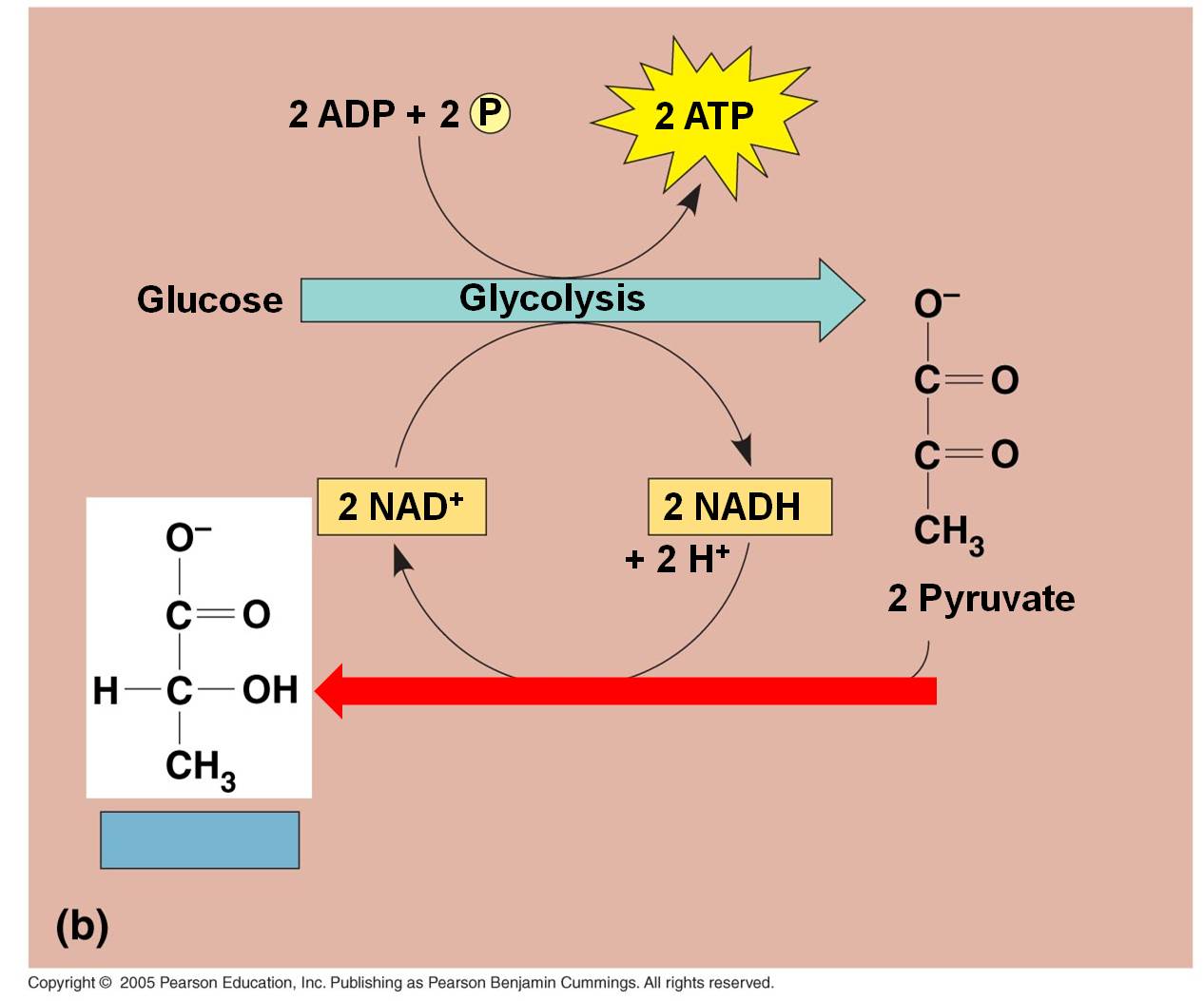 b.
In bacteria, fungi, and some animal cells:
b.
In bacteria, fungi, and some animal cells:
In lactic acid fermentation, the electron and H+ on NADH is transferred directly
to the C3 pyruvates. This converts them into lactate.
Typically, this type of respiration in animals can only occur for short periods
because the energetic demands for ATP will eventually exceed the rate of production
from glycolysis, alone. At this point, oxygen concentrations must rise
again so that the lactate can be converted back to pyruvate and metabolized
by aerobic respiration. In animal muscle cells, this is known as the 'oxygen
debt', and it is why animals keep breathing hard even after they have stopped
exerting themselves. Short, intensive bursts of activity, usually for up to
two minutes, will burn up available oxygen and cells will rapidly switch to
anaerobic pathways. And of course, the reason these intense activities can't
be sustained for more than 2 minutes at a time is that anaerobic pathways don't
produce enough energy to sustain it! So, you will only be able to run at maximum
speed for a short while... then you stop. Curiously, even though you have stopped
and are not exerting yourself any more, you still breathe heavily for a while.
You are pumping oxygen concentrations back up to normal levels, and processing
the lactate that has built up in your muscle cells. In the presence of oxygen,
the lactate can be convereted back into pyruvate, and then processed in the
reactions described below.
Anaerobic respiration and lactate fermentation break only a single carbon-carbon bond in the glucose molecule. As such, only a small amount of the energy present in a glucose molecule is released. In aerobic respiration, the glucose is completely oxidized - all six carbon atoms are broken apart, and much more energy is released (and harvested).
Oxygen is a very reactive gas - it oxidizes things - stripping electrons from other molecules and breaking bonds. Combustion is an oxidative process, and it can occur spontaneously, without an ignition source. So, if combustible material heats up above its ignition temperature, and if a strong oxidative agent like oxygen is present, it will ignite. Gasoline is a long hydrocarbon polymer. When raised above it's ignition point, it will combust. The gasoline will be oxidized to CO2 and H2O - and the breaking of the carbon-carbon bonds that occurs during this process will release energy. A gallon of gasoline contains ALOT of bonds and ALOT of energy; and if it is released all at once, the energy is difficult to control or use - you get an uncontrolled explosion. In a car's internal combustion engine, very small amounts of gasoline are ignited in sequence, by spark plugs, causing a little explosion in each cylinder that pushes the piston that turns the crankshaft that turns the wheels of the car. In a diesel engine, there are no spark plugs and no spark; the fuel ignites when the temperature exceeds its ignition point when placed under high pressure when the piston rises. By controling the reaction, by oxidizing just a little at a time, the energy released can be used to do work.
All organic molecules - sugars, nucleic acids, proteins, and fats - can be oxidized in the presence of a strong oxidative agent like oxygen gas. In fact, after oxygenic photosynthesis evolved and after iron precipitated out of suspension, when oxygen began to accumulate in the oceans and atmosphere, it was probably toxic to life. As described above, most of the most ancient forms of life - like archaeans - are obligate anaerobes and are still poisoned by oxygen to this day. Some life forms evolved specific enzymes and 'anti-oxidants' to protect themselves from the oxidative effects of oxygen gas, and they tolerated this new environment. Some of their descendants evolved a mechanism to use the oxidative effects of oxygen gas in a productive way - in a way that released the energy in organic molecules in controlled reactions where the energy could be harvested effectively and used to do chemical work. These aerobic organisms came to dominate the planet, probably in part because they could harvest more energy from the food they consumed; energy they could use to grow, survive, and reproduce. Aerobic respiration probably evolved about 2.0 billion years ago, in response to the increase in oxygen concentrations. Shortly thereafter, aerobically respiring bacteria were engulfed by other cells but not consumed; rather, the host cells used the ATP produced by these energetically efficient 'bacteria'. These host cells were the first eukaryotes, that evolved about 900 million years ago. Their energetic endosymbionts evolved into the mitochondria present in living eukaryotic cells. Like chloroplasts, mitochondria have their own bacteria-like chromosome and a bacteria-like double membrane system. In eukaryotes, the process of aerobic respiration occurs within these organelles. From these ancestral eukaryotes, some absorbed photosynthetic endosymbionts, too; these because the photosynthetic algae and their descendants, the plants. SO - PLEASE KNOW THIS: all eukaryotes (except a couple wierd protists like Giardia), HAVE MITOCHONDRIA - that includes protists, fungi, animals, and PLANTS. Photosynthetic eukaryotoes, the agae and plants, ALSO HAVE chloroplasts.
a. Overall Process:
- Pyruvates are broken down
into carbon dioxide.
- Energy that is released from
the complete breadown of the C-C bonds is used to make bonds in ATP (38).
- When bonds are broken, electrons
are released. They have to be accepted by another molecule. They are initally
accepted by NAD and FAD, which then take their "high energy" forms of NADH and
FADH2. Ultimately, they transfer this energy to ATP, give up
their electrons and H+, and are recylced as NAD and FAD. (That's important,
remember? We need to recycle that NAD so glycolysis - the first step in
this whole process - can continue.)
- Ultimately, the electrons
are passed to Oxygen O--, which then binds two hydrogen ions to balance charge
(forming water).
- Aerobic respiration is a
more complete breakdown of glucose, so it yields more ATP than glycolysis, alone
- In eukaryotes, this occurs
in a three step process in the mitochondria of cells.
 b.
Mitochondrial Structure:
b.
Mitochondrial Structure:
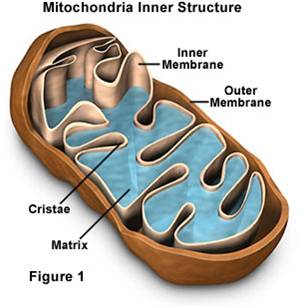
- Mitochondria have a double membrane
system like bacteria and chloroplasts, with an intermembrane space and matrix
within inner membrane.
- They have their own DNA, and they
replicate themselves by fission - they aren't 'made' by the cell.
- Given these observations, Lynn
Margulis hypothesized that these similarities were due to common ancestry, rather
than common environment. She raised this hypothesis as the endosymbiotic
hypothesis of eukaryote evolution, hypothesizing that eukaryotes acquired their
organelles by engulfing free-living bacteria and, rather than digesting them,
simply engulfed them and consumed their products (in this case the ATP that
the bacteria produce. The relationship is called symbiotic, because Margulis
hypothesized that the bacteria would also benefit by being in a stable environment
where the concentration of glucose was high (inside the cell).
- The most direct test of a hypothesis
of relatedness is DNA similarity. DNA only comes from parents, so similarities
imply a common source. When these tests were performed in the 1970's, her hypothesis
was confirmed. Additional tests with choloplasts and basal bodies (other
organielles in eukaryotes) also showed strong patterns of relatedness with free-living
bacteria. As such, we now refer to this tested model as the Endosymbiotic
Theory. We will describe this theory in more detail later in the term...
c. The Details:
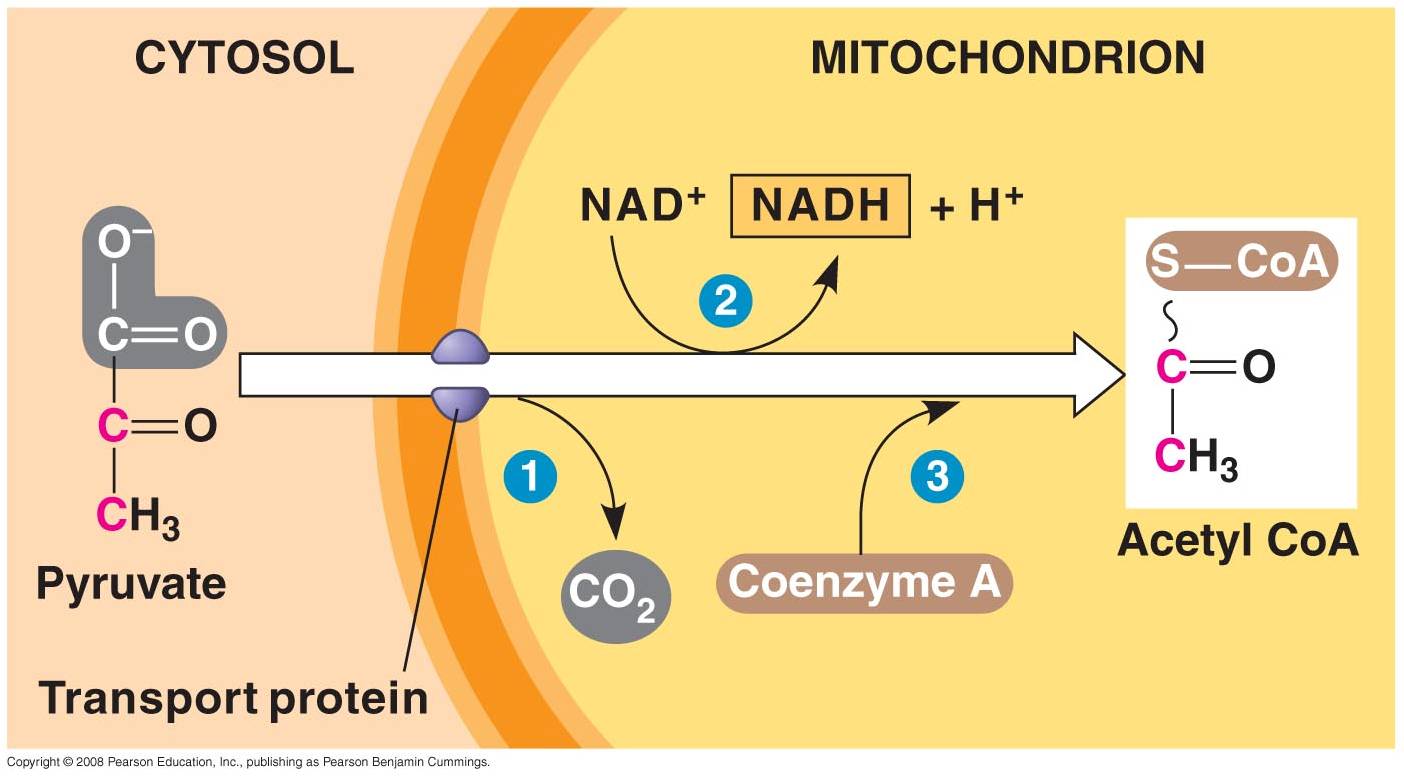 1. 'Gateway' Step:
1. 'Gateway' Step:
- Pyruvates cross both membranes
into the mitochondria and enter the 'matrix' - the cytoplasm of the organelle.
- Each pyruvate reacts with
a Coenzyme A molecule, and is split into a C2-CoA molecule and CO2.
(One C broken off).
- The electrons and energy
released are accepted by NAD, forming 1 NADH for each pyruvate used.
2. Krebs Cycle: 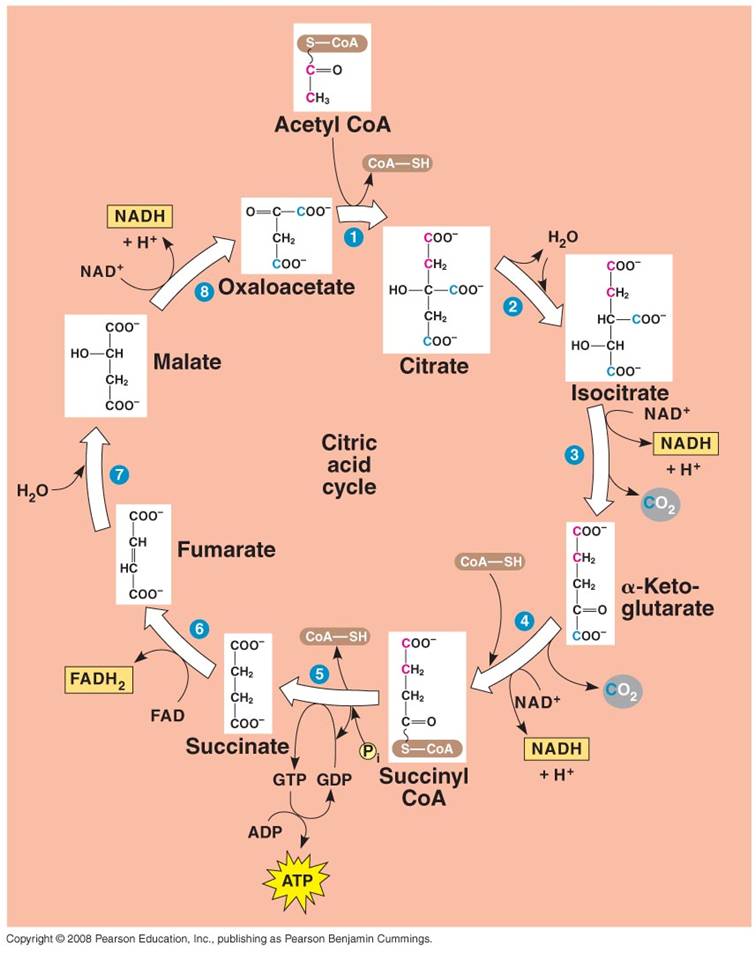
- Each C2-CoA reacts
with a C4 molecule (oxaloacetate).
- The C2 acetate
is transferred to the C4 molecule, forming a C6 molecule
of citrate. (CoA is released and recycled).
- Through a series of reactions,
the 2 'extra' C's are broken off as CO2 molecules and the C4
molecule is regenerated (Cycle).
- Some of the energy released
by the breaking of the C-bonds is used to make 1 ATP, 3 NADH, and 1 FADH2.
3. Electron Tranport Chain:
- Proteins nested in the inner
membrane of the mitochondria accept the electrons from NADH and FADH2
- The electrons are passed
from molecule to molecule, and some of the energy released is used to pump H+
ions across the inner membrane from the matrix to the intermembrane compartment
- Asteep concentration gradient
of H+ ions is formed... this represents chemical potential energy.
- When the ions flow through
protein channels associated with ATP-synthesizing enzymes in the membrane, this
potential energy is transformed into chemical energy in bonds between ADP and
P, making ATP.
- When the electrons reach
a low energy state, they are accepted by oxygen in the matrix and H+ ions react
with the O-- to form water. This is the ONLY use of oxygen in the process
- as an electron acceptor.
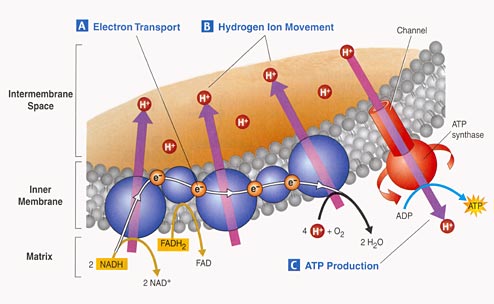
- 34 to 36 ATP are made from the energy tranferred from NADH and FADH2 molecules produced in the Krebs Cycle.
a.
Fats:
- Glycerol broken from fatty acids; glycerol (3C) fed into glycolysis and
are modified into pyruvates (C3). - the Fatty Acids are broken
down into C2 groups that are modified to react with CoA - they
are shuinted to the Krebs Cycle - these reacts are reversible, so if there
is a surplus of C2-CoA, it can react to form fatty acids ---> energy
consumed in carbo's can be stored as bonds in fat.
b.
Proteins:
- Broken into Amino Acids which, depending on their structure, can be shunted
into glycolysis, modified into pyruvate, or broken into acetate (C2).
- In all cases, the amine groups are cleaved, producing ammonia (NH3)
as a toxic waste. In mammals, this is converted into urea which must be diluted
in water for removal from the body (urine). Reptiles and birds convert it
to uric acid, which is expelled as a paste that does not require as much water
for dilution.
c.
Nucleic Acids: - ribose can be metabolized after coversion to
glucose.
Study Questions:
1. How can biological systems get bigger and more complex without violating the second law of thermodynamics?
2. Describe glycolysis.
3. What is the purpose of fermentation, and under what environmental condition does it occur?
4. What happens in the gateway step?
5. What happens in the krebs cycle?
6. What happens in the elctron transport chain? Explain chemiosmosis.
7. How is oxygen involved in the process of aerobic respiration?