Cell Biology
I. Overview
II. Membranes: How
Matter Get in and Out of Cells
III. DNA, RNA, and
Chromosome Structure
IV. Protein Synthesis
V. Origin
of Life Hypotheses
VI.
Photosynthesis
Overview:
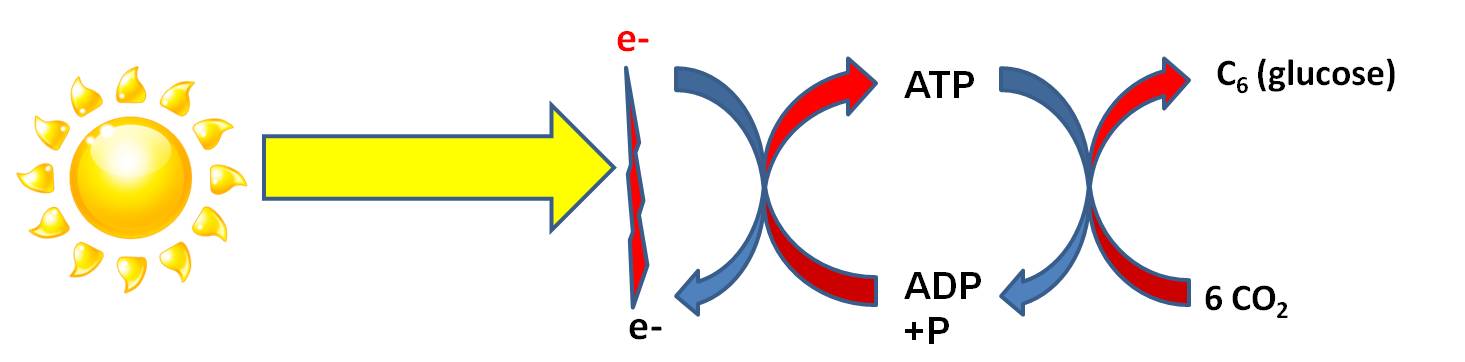 Photosynthesis
is a process of energy transformation. Again, although energy can neither be
created nor destroyed, it can be transformed. In the "Light Dependent Reaction"
radiant energy ('carried' by photons in light) is transformed into chemical
energy ('carried' by electrons). It requires an electron DONOR to provide electrons
that will 'carry' this energy. The energy 'carried' by this electron is used
to form a bond between ADP and P, creating ATP. Through this transfer, the electron
loses this energy. As we have discussed before, the phosphate bonds in ATP are
easily made and easily broken - that's why energy in this form of chemical bond
can be 'used' by all enzymes in the cell. However, ATP is readily hydrolyzed
in water...so it is difficult for a cell to build up a large amount of ATP before
it 'dissolves' to ADP and P again. To store large amounts of energy for a longer
time, the energy in ATP can be converted to a more stable molecule. In most
photosynthetic organisms, the catabolism of ATP is coupled to anabolic reactions
that bind carbon dioxide molecules together into stable molecules of glucose,
for longer term E storage. This also provides the cell with organic carbon that
it can use to make the other biologically important molecules. These are the
"Light Independent Reactions" of photosynthesis.
Photosynthesis
is a process of energy transformation. Again, although energy can neither be
created nor destroyed, it can be transformed. In the "Light Dependent Reaction"
radiant energy ('carried' by photons in light) is transformed into chemical
energy ('carried' by electrons). It requires an electron DONOR to provide electrons
that will 'carry' this energy. The energy 'carried' by this electron is used
to form a bond between ADP and P, creating ATP. Through this transfer, the electron
loses this energy. As we have discussed before, the phosphate bonds in ATP are
easily made and easily broken - that's why energy in this form of chemical bond
can be 'used' by all enzymes in the cell. However, ATP is readily hydrolyzed
in water...so it is difficult for a cell to build up a large amount of ATP before
it 'dissolves' to ADP and P again. To store large amounts of energy for a longer
time, the energy in ATP can be converted to a more stable molecule. In most
photosynthetic organisms, the catabolism of ATP is coupled to anabolic reactions
that bind carbon dioxide molecules together into stable molecules of glucose,
for longer term E storage. This also provides the cell with organic carbon that
it can use to make the other biologically important molecules. These are the
"Light Independent Reactions" of photosynthesis.
 When
we think of photosynthesis, most of us think "plants". This is generally
correct, but very incomplete. First, there are some plants like Indian Pipe
(Monotropa uniflora) that do not photosynthesize. Although they evolved
from photosynthetic ancestors, they have adopted a parasitic lifestyle and no
longer harvest their own energy from sunlight. In addition, there are photosynthetic
protists (algae and Euglenozoans), and photosynthetic archaeans and eubacteria.
In fact, there are several animals that harbor photosynthetic symbionts, too.
Many corals (corals are animals) ingest algal cells and distribute them to their
tentacles. The algae photosynthesize, and excess sugars are passed to the coral
animal. These symbiotic algae give corals their spectacular colors. When stressed
by water polution or high water temperatures, the corals release their symbionts
and lose their color ("a phenomenon called "coral bleaching").
Long periods without their symbionts results in coral death.
When
we think of photosynthesis, most of us think "plants". This is generally
correct, but very incomplete. First, there are some plants like Indian Pipe
(Monotropa uniflora) that do not photosynthesize. Although they evolved
from photosynthetic ancestors, they have adopted a parasitic lifestyle and no
longer harvest their own energy from sunlight. In addition, there are photosynthetic
protists (algae and Euglenozoans), and photosynthetic archaeans and eubacteria.
In fact, there are several animals that harbor photosynthetic symbionts, too.
Many corals (corals are animals) ingest algal cells and distribute them to their
tentacles. The algae photosynthesize, and excess sugars are passed to the coral
animal. These symbiotic algae give corals their spectacular colors. When stressed
by water polution or high water temperatures, the corals release their symbionts
and lose their color ("a phenomenon called "coral bleaching").
Long periods without their symbionts results in coral death.
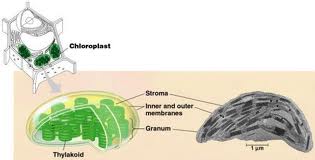 Photosynthesis
in prokaryotes occurs on the double-membrane system of these organisms. In eukaryotes,
photosynthesis occurs in organelles called chloroplasts. Chloroplasts have a
bacteria-like double membrane, and they have their own DNA. This DNA is more
similar in most respects to the DNA in free-living bacteria than to the DNA
in the nucleus of the eukaryotic cells they 'inhabit'. For these reasons, most
scientists accept the 'endosymbiotic theory' of chloroplast origins. This theory
states that chloroplasts in the cells of photosynthetic eukaryotes are descendants
of free-living photosynthetic bacteria. At some point in the early evolution
of protists, these photosynthetic bacteria were engulfed by not digested. Rather,
the host cells fed on the excess sugars produced by the internalized bacteria.
Eventually, as the result of gene exchange between the host and proto-chloroplasts,
the eukaryotic host and the prokaryotic symbiont became dependent on one another.
But chloroplasts can still live outside of cells for several days. Plants, evolving
from green algae ancestors, inherited these bacteria-like chloroplasts, too.
Photosynthesis
in prokaryotes occurs on the double-membrane system of these organisms. In eukaryotes,
photosynthesis occurs in organelles called chloroplasts. Chloroplasts have a
bacteria-like double membrane, and they have their own DNA. This DNA is more
similar in most respects to the DNA in free-living bacteria than to the DNA
in the nucleus of the eukaryotic cells they 'inhabit'. For these reasons, most
scientists accept the 'endosymbiotic theory' of chloroplast origins. This theory
states that chloroplasts in the cells of photosynthetic eukaryotes are descendants
of free-living photosynthetic bacteria. At some point in the early evolution
of protists, these photosynthetic bacteria were engulfed by not digested. Rather,
the host cells fed on the excess sugars produced by the internalized bacteria.
Eventually, as the result of gene exchange between the host and proto-chloroplasts,
the eukaryotic host and the prokaryotic symbiont became dependent on one another.
But chloroplasts can still live outside of cells for several days. Plants, evolving
from green algae ancestors, inherited these bacteria-like chloroplasts, too.
 Photosynthesis
is a critically important process in the evolution and diversity of life. Prior
to the evolution of photosynthesis, life was dependent on absorbing spontaneously
generated organic molecules, or preying on other cells. Neither of these sources
of energy was probably all that common and easy to find. Evolving the ability
to use sunlight as an energy source, which IS abundant and IS easy to find,
meant that life could grow, prosper, and radiate dramatically - almost anywhere
there was a light source. Indeed, it looks like photosynthesis evolved very
early in the history of life; the earliest fossils (stromatolites and filamentous
microfossils dating to ~3.5 by) look very similar to photosynthetic bacteria
that are alive today. When photosynthetic organisms became abundant, they provided
a food supply for a wider variety of heterotrophic cells. Heterotrophs could
then live anywhere phototrophs lived; they were not limited to those rare places
where biological molecules were forming spontaneously. So, complex bacterial
food webs evolved. These early photosynthetic organisms used a primitive form
of photosynthesis that did not produce oxygen as a waste product. So, even though
they flourished for a billion years, no oxygen was added to the atmosphere.
About 2.0 billion years ago, a 'modern' type of photosynthesis evolved that
used water as the electron donor and produced oxygen gas as a waste product.
The production of oxygen gas transformed the oceans (precipitating iron), and
eventually changed the atmosphere, as well. Although oxygen was probably a highly
toxic gas at first (because it is so reactive), life eventually evolved to tolerate
it and then to USE it in oxidative respiration. The evolution of aerobic respiration
allowed for more energy to be harvested from the catabolism of complex organic
molecules, and may have allowed for the evolution of more energy-demanding eukaryotes
and multicellular organisms. As you know, almost all food webs are ultimately
dependent on the photosynthetic organisms at the base of the "food chain"
(hydrothermal vent communities are a possible exception). We use this energy
to stick amino acids together to make our proteins, etc. Even the gas and oil
that powers our industrial societies was initally stored as glucose produced
by photosynthesis. Coal, gas, and oil are just fossilized plants - and we "burn"
that energy millions of years after it was converted from sunlight. We are powering
our societies with sunlight that hit the Earth millions of years ago. But not
only are you (and every other heterotroph) energetically dependant on photosynthetic
organisms for food, you are also indebted to them for changing the planet and
stimulating the evolution of eukaryotic and multicellular life. In short, there
are few processes more important to the history and current function of living
systems (and our petroleum-based economy) than photosynthesis.
Photosynthesis
is a critically important process in the evolution and diversity of life. Prior
to the evolution of photosynthesis, life was dependent on absorbing spontaneously
generated organic molecules, or preying on other cells. Neither of these sources
of energy was probably all that common and easy to find. Evolving the ability
to use sunlight as an energy source, which IS abundant and IS easy to find,
meant that life could grow, prosper, and radiate dramatically - almost anywhere
there was a light source. Indeed, it looks like photosynthesis evolved very
early in the history of life; the earliest fossils (stromatolites and filamentous
microfossils dating to ~3.5 by) look very similar to photosynthetic bacteria
that are alive today. When photosynthetic organisms became abundant, they provided
a food supply for a wider variety of heterotrophic cells. Heterotrophs could
then live anywhere phototrophs lived; they were not limited to those rare places
where biological molecules were forming spontaneously. So, complex bacterial
food webs evolved. These early photosynthetic organisms used a primitive form
of photosynthesis that did not produce oxygen as a waste product. So, even though
they flourished for a billion years, no oxygen was added to the atmosphere.
About 2.0 billion years ago, a 'modern' type of photosynthesis evolved that
used water as the electron donor and produced oxygen gas as a waste product.
The production of oxygen gas transformed the oceans (precipitating iron), and
eventually changed the atmosphere, as well. Although oxygen was probably a highly
toxic gas at first (because it is so reactive), life eventually evolved to tolerate
it and then to USE it in oxidative respiration. The evolution of aerobic respiration
allowed for more energy to be harvested from the catabolism of complex organic
molecules, and may have allowed for the evolution of more energy-demanding eukaryotes
and multicellular organisms. As you know, almost all food webs are ultimately
dependent on the photosynthetic organisms at the base of the "food chain"
(hydrothermal vent communities are a possible exception). We use this energy
to stick amino acids together to make our proteins, etc. Even the gas and oil
that powers our industrial societies was initally stored as glucose produced
by photosynthesis. Coal, gas, and oil are just fossilized plants - and we "burn"
that energy millions of years after it was converted from sunlight. We are powering
our societies with sunlight that hit the Earth millions of years ago. But not
only are you (and every other heterotroph) energetically dependant on photosynthetic
organisms for food, you are also indebted to them for changing the planet and
stimulating the evolution of eukaryotic and multicellular life. In short, there
are few processes more important to the history and current function of living
systems (and our petroleum-based economy) than photosynthesis.
A. Step 1: The Light Dependent Reaction
AGAIN, the purpose of the light-dependent reaction is to convert radiant energy
to chemical energy. Obviously, light must be present; so this reaction "depends"
on sunlight. There is one
group of Archaeans that performs photosynthesis (Halobacteria), but their process
of harvesting light energy seems quite different from the process in eubacteria
and chloroplasts in eukarya and probably evolved independently. Within the eubacteria,
there are also a wide variety of photosynthetic processes. We will focus on
a couple major types and make reference to others as we go.
1. PRIMITIVE
SYSTEMS:
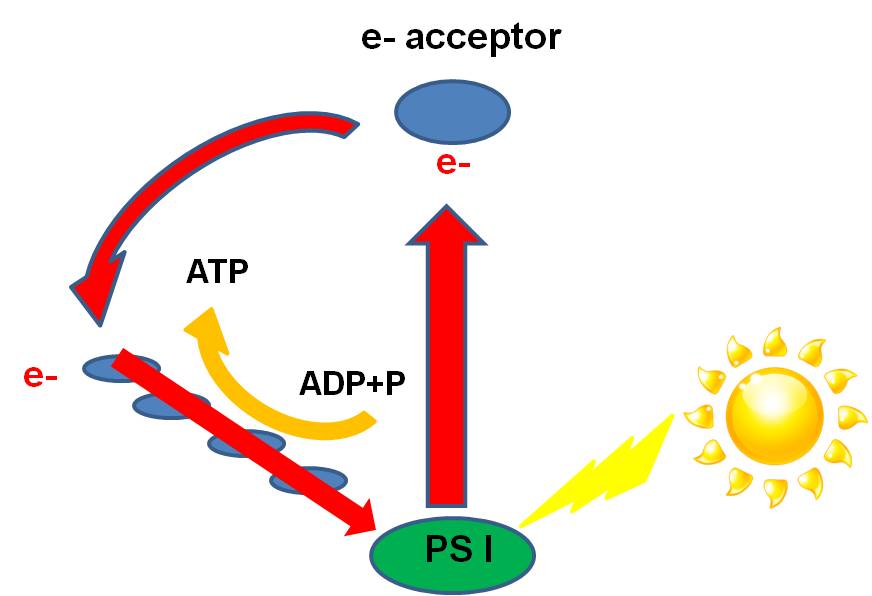 a.
cyclic phosphorylation in "purple non-sulphur" and "green non-sulpher"
bacteria:Like all
bacteria, they have a double membrane (two bilayers). Proteins nested within
the inner membrane form "reaction centers (also called "photosystems")
and "electron transport chains" (ETC's) used in photosynthesis. This
inner membrane is often highly convoluted, increasing the surface area and the
number of reaction centers and ETC's that can be imbedded. Each reaction
center contains proteins arrayed around molecules of bacteriochlorophyll, which
contain atoms of Magnesium. In the
presence of light, the photons transfer energy to these electrons. The electrons
are raised to a higher energy state, lost from
the atom, and transferred to an 'electron acceptor molecule' in the inner membrane
of the bacterium, which transfers the electron the the electron transport chain.
When a high-energy electron
is transferred down the chain, protons
(H+) follow ('electrostatically') and are pumped across the inner membrane into
the intramembrane space. This build-up of H+ ions in the intermembrane space
creates an electrostatic charge differential across the membrane. There are
closed protein channels that, when opened, allow the H+ to flood through in
response to the charge gradient. This electric discharge energy is used by the
enzyme ATP-synthetase to add a phosphate group to ADP, making ATP. This is called
'chemiosmotic synthesis' or 'chemiosmosis'. So, what has happened is that the
passage of an electron -excited by light energy - has been used to 'pump protons'
into the intermembrane space, establishing an H+ ion charge gradient. The flow
of H+ ions through protein channels transforms this electric energy to chemical
bond energy in the form of a bond between ADP and P--> ATP. The
high-energy electron is then passed down the electron transport chain. and ATP
is produced. The electron, having lost its energy, can be recycled back to the
Mg atom. This cyclic production of ATP, powered by sunlight, is called cyclic
phosphorylation. As discussed below, these odd bacteria do not perform the light
independent pathways. In other words, they do not use the energy in ATP to make
glucose. This has two interesting consequences. First, it means they can't rely
on photosynthesis, alone, for energy harvest, because ATP isn't stable enough
to last over the course of an evening. So, they must also 'eat' - they are heterotrophs,
and can harvest energy from the food they ingest. The other consequence is discussed
below.
a.
cyclic phosphorylation in "purple non-sulphur" and "green non-sulpher"
bacteria:Like all
bacteria, they have a double membrane (two bilayers). Proteins nested within
the inner membrane form "reaction centers (also called "photosystems")
and "electron transport chains" (ETC's) used in photosynthesis. This
inner membrane is often highly convoluted, increasing the surface area and the
number of reaction centers and ETC's that can be imbedded. Each reaction
center contains proteins arrayed around molecules of bacteriochlorophyll, which
contain atoms of Magnesium. In the
presence of light, the photons transfer energy to these electrons. The electrons
are raised to a higher energy state, lost from
the atom, and transferred to an 'electron acceptor molecule' in the inner membrane
of the bacterium, which transfers the electron the the electron transport chain.
When a high-energy electron
is transferred down the chain, protons
(H+) follow ('electrostatically') and are pumped across the inner membrane into
the intramembrane space. This build-up of H+ ions in the intermembrane space
creates an electrostatic charge differential across the membrane. There are
closed protein channels that, when opened, allow the H+ to flood through in
response to the charge gradient. This electric discharge energy is used by the
enzyme ATP-synthetase to add a phosphate group to ADP, making ATP. This is called
'chemiosmotic synthesis' or 'chemiosmosis'. So, what has happened is that the
passage of an electron -excited by light energy - has been used to 'pump protons'
into the intermembrane space, establishing an H+ ion charge gradient. The flow
of H+ ions through protein channels transforms this electric energy to chemical
bond energy in the form of a bond between ADP and P--> ATP. The
high-energy electron is then passed down the electron transport chain. and ATP
is produced. The electron, having lost its energy, can be recycled back to the
Mg atom. This cyclic production of ATP, powered by sunlight, is called cyclic
phosphorylation. As discussed below, these odd bacteria do not perform the light
independent pathways. In other words, they do not use the energy in ATP to make
glucose. This has two interesting consequences. First, it means they can't rely
on photosynthesis, alone, for energy harvest, because ATP isn't stable enough
to last over the course of an evening. So, they must also 'eat' - they are heterotrophs,
and can harvest energy from the food they ingest. The other consequence is discussed
below.
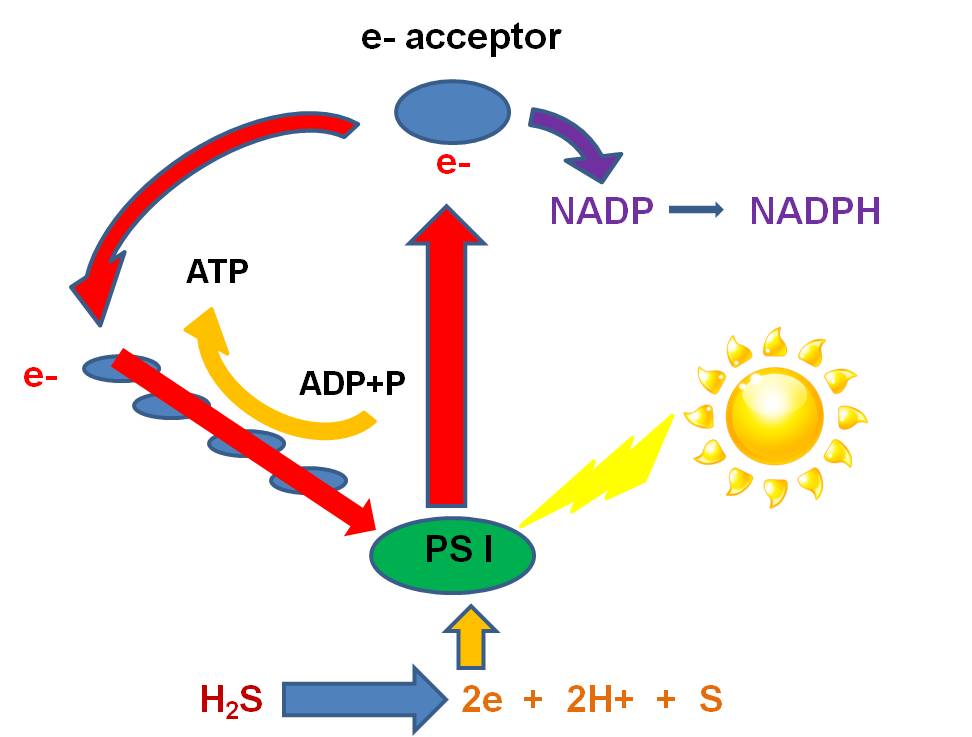 b.
"green sulphur" and "purple
sulphur" bacteria that
use sulphides as the electron donors:
b.
"green sulphur" and "purple
sulphur" bacteria that
use sulphides as the electron donors:
When the excited electron is recieved by the 'electron acceptor',something else
can happen. Instead of the Electron Acceptor giving the electron to the ETC,
it can give the electron to NADP... another 'energy transport molecule' like
ADP. When this happens, the NADP gains energy and a negative charge and is NADP-.
It reacts with free H+ ions that are always present in aqueous solutions (you
should know why...), to make the high energy transport molecule, NADPH. In this
case, the electron isn't returned to the Magnesium.... photosynthesis would
stop, unless the photosystem can strip electrons from other molecules in solution.
There are several groups of primitive
eubacteria ("green sulphur bacteria" and "purple sulphur bacteria")
that use sulfides (like hydrogen sulfide - H2S) as the electron donor.
Sulphur bacteria have photosystems
that strip electrons from Hydrogen Sulphide (H2S). This releases
2H+ ions and S as a waste product. So, sulphur bacteria that are still present
today photosynthesize in sulphur springs and do not produce oxygen as a waste
product. This explains an interesting geological pattern: The oldest fossil
life on record are photosynthetic bacteria that date to 3.8 billion years old.
However, the first evidence of oxygen in the Earth's atmosphere occurs at about
2 billion years ago. So, how can you now explain how there were photosynthetic
bacteria present for 1.8 billion years, without any oxygen being produced? Sulphur
bacteria. And they have another interesting characteristic - they are anaerobic
organisms poisoned by oxygen gas. So, not only don't they produce oxygen, but
they can only survive in its absence. All these factors suggest that they may
be similar to the first photosynthetic life forms that thrive in the anaerobic
environment of the early earth. There is a problem for them, however. These
bacteria can only survive in places where H2S is abundant - like
sulphur springs. These places are rare. If something evolved a system that could
strip electrons from a more abundant source, like water (H2O), then
these new organisms could exploit almost the whole planet - as 75% of the planet
is covered by H2O.
2. ADVANCED
SYSTEM: most
other photosynthetic bacteria (cyanobacteria), and photosynthetic eukaryotes.
- In photosynthetic Eukaryotes(photosynthetic protists and plants), these reactions
occur on the inner membrane of the Chloroplast - a specific membrane-bound organelle
very much like a bacterium within the larger eukaryotic cell. Indeed, as described
above, eukaryotic chloroplasts are probably the deescendants of free-living
cyanobacteria - with whom they share basic membrane structure and DNA similarity.
- In cyanobacteria and chloroplasts, there
are two types of reaction centers called "photosystems". The second
photosystem (PSII) has a lower electronegativity than the first, so it can exert
a 'stronger' pull and can strip electrons from WATER (which holds the electrons
more strongly than H2S does.) The splitting of water releases oxygen
gas as a waste product, so this type of photosynthesis is also called "oxygenic
photosynthesis".
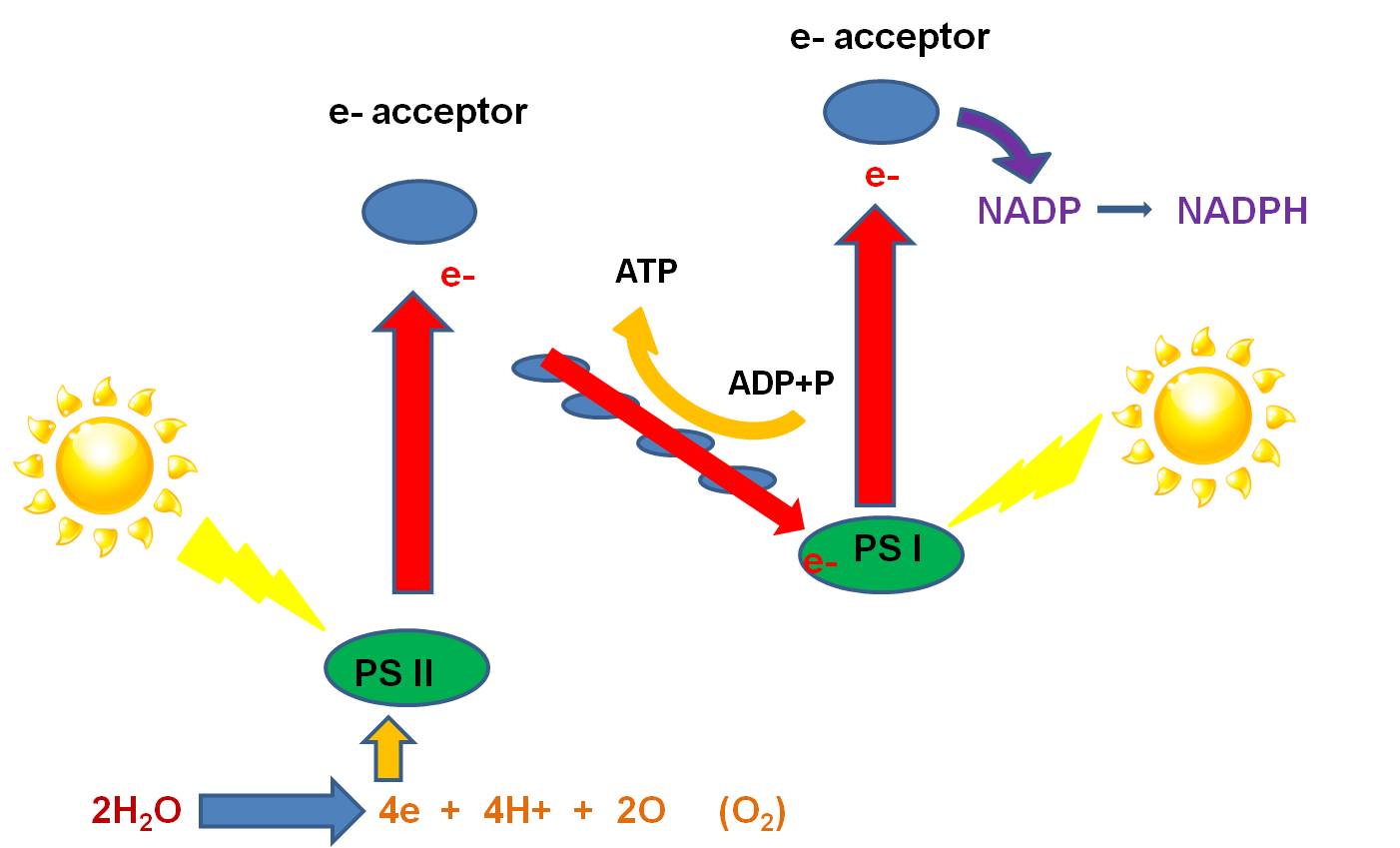 - Here's how it works: Light strikes the phosystems nested in the inner membrane
(called the 'thylakoid' membrane in chloroplasts). An electron in each photosystem
is excited and lost from the Mg in the chlorophyll molecule. The electrons are
accepted by partcular electron acceptor molecules. The electron lost from PS
I is ultimately passed to NADP, which accepts a H+ to balance the charge, making
the high energy molecule, NADPH. The electron lost from PSII is passed to an
electron acceptor, and then to molecules in the electron transport chain. As
the electron is passed down the chain, ATP is produced by chemiosmosis (as described
above). When this electron has lost it's energy, it replaces the electron lost
from PS I. So, PS I is all set, and need not strip electrons from an electron
donor. However, PS II has lost an electron, and must replace this electron for
photosynthesis to continue. PSII strips electrons from H2O. Water
is split into oxygen, 2 H+, and 2 electrons. The electrons are passed to the
cholorophyll in PS II, excited by light, and energized. The oxygen reacts with
another oxygen atom to produce oxygen gas, which is released as a waste product.
The propose of photosynthesis is not "to produce oxygen". The purpose
of the light reaction of photosynthesis is to transform radiant energy into
chemcial energy, and produce ATP and NADPH. The two molecules, ATP and NADPH,
are the useful products. Again, oxygen gas is produced as a waste product when
electrons are stripped from water. The presence of oxygen in the oceans 2.5-2
billion years ago, indicated by the presence of sedimentary deposits with oxidized
iron (banded iron formations), indicates the evolution of this more advanced
type of photosynthesis that evolved in ancient photosynthetic bacteria.
- Here's how it works: Light strikes the phosystems nested in the inner membrane
(called the 'thylakoid' membrane in chloroplasts). An electron in each photosystem
is excited and lost from the Mg in the chlorophyll molecule. The electrons are
accepted by partcular electron acceptor molecules. The electron lost from PS
I is ultimately passed to NADP, which accepts a H+ to balance the charge, making
the high energy molecule, NADPH. The electron lost from PSII is passed to an
electron acceptor, and then to molecules in the electron transport chain. As
the electron is passed down the chain, ATP is produced by chemiosmosis (as described
above). When this electron has lost it's energy, it replaces the electron lost
from PS I. So, PS I is all set, and need not strip electrons from an electron
donor. However, PS II has lost an electron, and must replace this electron for
photosynthesis to continue. PSII strips electrons from H2O. Water
is split into oxygen, 2 H+, and 2 electrons. The electrons are passed to the
cholorophyll in PS II, excited by light, and energized. The oxygen reacts with
another oxygen atom to produce oxygen gas, which is released as a waste product.
The propose of photosynthesis is not "to produce oxygen". The purpose
of the light reaction of photosynthesis is to transform radiant energy into
chemcial energy, and produce ATP and NADPH. The two molecules, ATP and NADPH,
are the useful products. Again, oxygen gas is produced as a waste product when
electrons are stripped from water. The presence of oxygen in the oceans 2.5-2
billion years ago, indicated by the presence of sedimentary deposits with oxidized
iron (banded iron formations), indicates the evolution of this more advanced
type of photosynthesis that evolved in ancient photosynthetic bacteria.
3. SUMMARY
OF LIGHT REACTIONS:
- Radiant energy excites electrons,
which are passed down an electron transport chain and ATP is made.
- The first organisms to evolve
this process probably used H2S as the donor of these electrons.
These sulphur bacteria do not produce oxygen as a waste product; they produce
sulphur.
- The evolution of PSII was very
important, because photosynthetic organisms could use H2O as the
electron donor and were no loner limited to living in places where H2S
was abundant. These organisms that use water as the electron donor produce
oxygen as a waste product, and the geologic record suggests that this process
evolved about 2.3-2.0 bya.
- Ultimately, the electrons are
passed to NADP, making NADPH.
- Water + ADP + NADP --> in the
presence of light --> ATP + NADPH + O2 (waste)
B. Step
2: The Light Independent Reactions:
The purpose of the "Light Independent
Reactions" is to convert the chemical energy in fragile ATP and NADPH molecules
into a more stable energy form by building covalent bonds between carbon atoms
to make glucose. In prokaryotes, these reactions occur in the cytoplasm of the
cell; in eukaryotes, these reactions occur in the stroma - or cytoplasm - of
the chloroplasts. It is important to appreciate that organisms using both primitive
and advanced light reactions perform the light independent reactions.
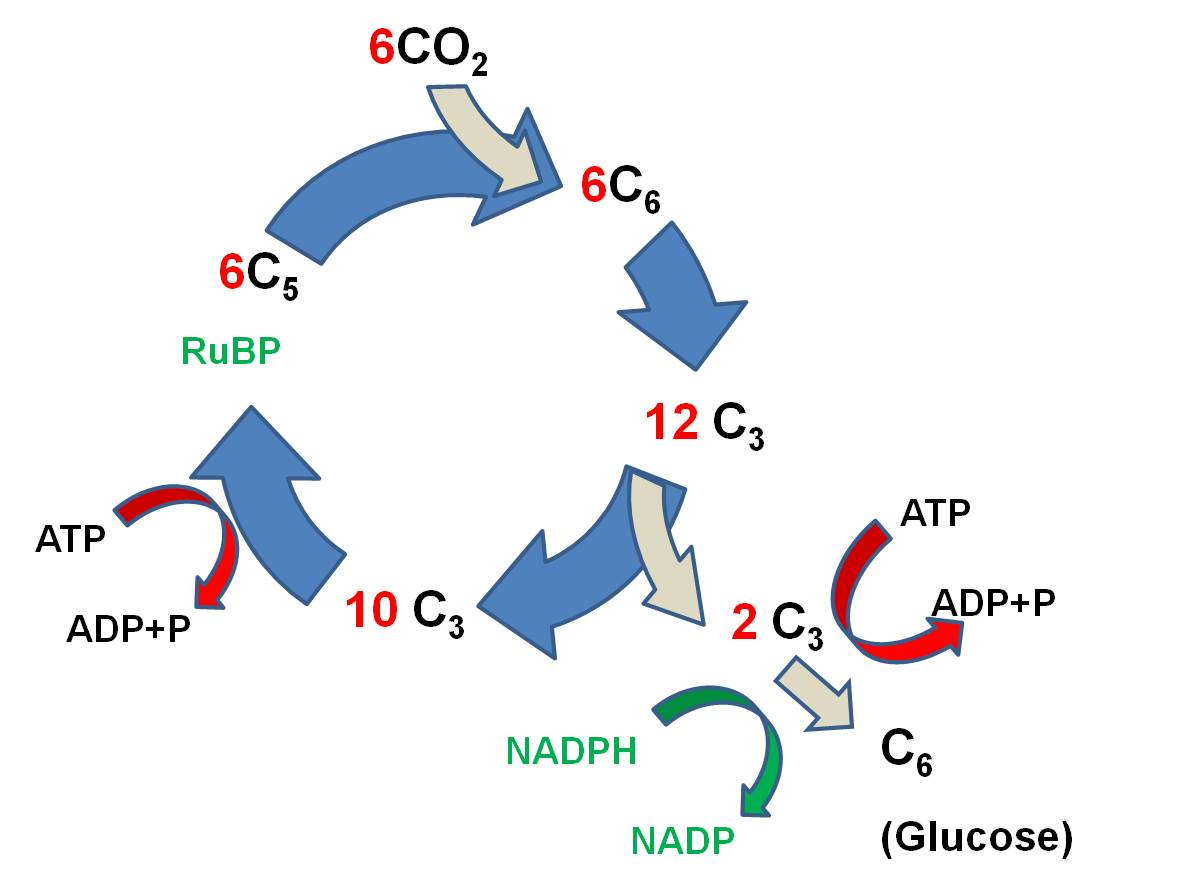 The
primary reaction is called the Calvin-Benson
Cycle, and it works like this:
The
primary reaction is called the Calvin-Benson
Cycle, and it works like this:
- 6 CO2 molecules bind
to 6 C5 molecules of Ribulose Biphosphate (RuBP), making 6 C6
molecules. (ATP is broken and the energy that is released is used to link
CO2 to RUBP).
- These energized C6 molecules
are unstable; the split into 12 C3 molecules. So, since the first
stable product is a C3 molecule, this type of reaction is called the C3 pathway.
- 2 C3 molecules are used
to form 1 glucose (C6) molecule. More ATP is used, and NADPH is used,
too, and H is transferred to put the 'hydrogen' in 'carbohydrate'.
- the 10 remaining C3
molecules (30 C total) are rearranged, using ATP and NADPH, and 6 C5
molecules are generated (30 C total).
The reaction can be summarized like
this: Six
CO2 molecules are used to make one molecule of glucose. Six RuBP
molecules are involved, and are recycled through the process. The ATP and NADPH
formed in the light reaction are used to power this reaction; the energy in
these molecules is used top make bonds between the CO2, and the H
from NADPH is used to reduce the CO2 to form glucose (C6H12O6).
As such, the radiant energy initially trapped in chemical bonds in ATP and NADPH
is transferred to form bonds between carbon atoms in glucose. The energy intially
trapped in fragile molecules has been stored in a more stable form.
When cells build glucose from CO2,
they have not only stored energy in a stable form - they have also harvested
carbon from the environment and transformed it into a usable organic molecule.
Since all biologically important molecules (except water) are carbon-based organic
molecules, all life forms needs a source of carbon to build amino acids, nucleotides,
sugars, and lipids. "Heterotrophs" get organic carbon in the 'food'
they eat. "Autotrophs" get their carbon through the light independent
reaction, which also stores energy.
The first group of bacteria discussed
above - the green non-sulphur bacteria and purple non-sulphur bacteria - perform
the Light Dependent Reaction and make ATP using sunlight, but they do not perform
the light indepedent reactions. So, they do not absorb CO2 to make their organic
molecules. Instead, they must consume organic molecules to acquire their carbon.
These organisms are "photoheterotrophs". They may represent the first
step in the evolution of photosynthesis: the evolution of light-trapping reactions
by heterotrophic cells. They use cyclic phosphorylation to make ATP in the presence
of light, but they use organic molecules as electron donors.
C. Photorespiration
- Problem
and Solutions:
1. PROBLEM:
 RuBP
will bind to BOTH CO2 and O2. And when RuBP binds
to O2, it is split and transformed to the amino acid serine, with
the production of CO2 as waste. Essentially, it is digested.
These reactions use the ATP and NADPH produced in the light reaction, too. So,
when RuBP binds O2, it does the exact opposite of photosynthesis
- it IS RESPIRED - the energy of the light reaction is used to BREAK DOWN a
carbohydrate (RuBP) and RELEASE CO2. This happens when the relative
concentrations of O2 and CO2 cross a critical threshold,
If O2 is super-abundant and CO2 is scarce, then photorespiration
will ocur. Oxygen levels can rise to these levels on HOT, DRY days, when there
is a lot of sun for photosynthesis to proceed (so O2 concentrations
rise in the leaf and CO2 concentrations fall), and it is DRY so the
stomates are closed and gases can't be exchanged with the environment (so O2
builds up in the leaf). It may seem curious that such a critical molecule has
such a debilitating characteristic. However, because
RuBP
will bind to BOTH CO2 and O2. And when RuBP binds
to O2, it is split and transformed to the amino acid serine, with
the production of CO2 as waste. Essentially, it is digested.
These reactions use the ATP and NADPH produced in the light reaction, too. So,
when RuBP binds O2, it does the exact opposite of photosynthesis
- it IS RESPIRED - the energy of the light reaction is used to BREAK DOWN a
carbohydrate (RuBP) and RELEASE CO2. This happens when the relative
concentrations of O2 and CO2 cross a critical threshold,
If O2 is super-abundant and CO2 is scarce, then photorespiration
will ocur. Oxygen levels can rise to these levels on HOT, DRY days, when there
is a lot of sun for photosynthesis to proceed (so O2 concentrations
rise in the leaf and CO2 concentrations fall), and it is DRY so the
stomates are closed and gases can't be exchanged with the environment (so O2
builds up in the leaf). It may seem curious that such a critical molecule has
such a debilitating characteristic. However, because
the light independent reaction probably evolved long before oxygenic light reactions,
it was already incorporated into the process before the accumulation of oxygen
in the oceans and atmosphere revealed its weakness.
2. SOLUTIONS:
a. C4 metabolism:
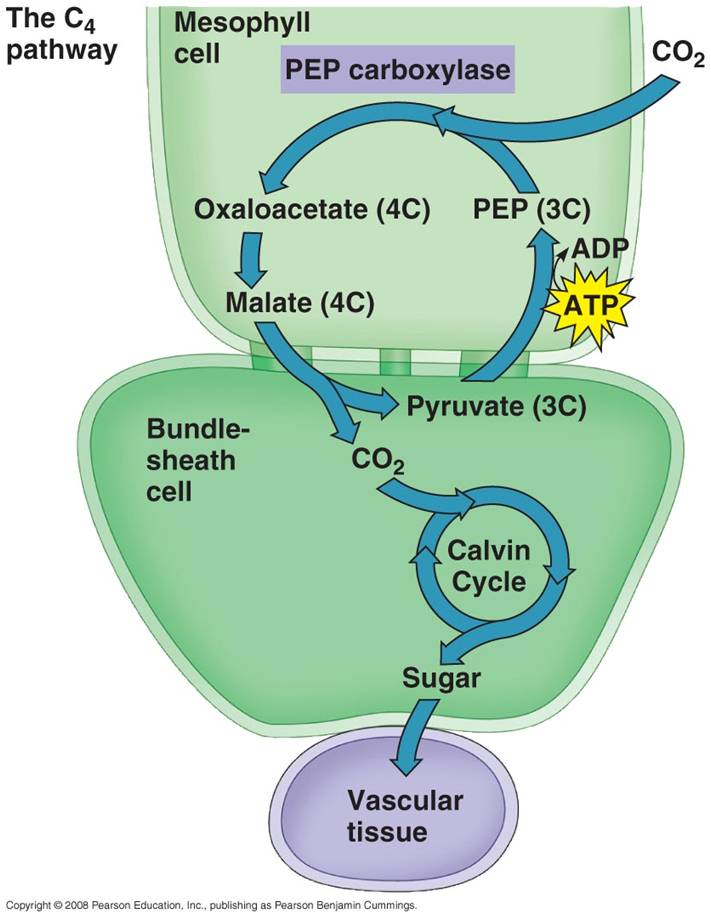 "C3
plants" (as described above - called C3 because the first STABLE product of
carbon fixation is a C3 molecule) have chloroplasts in their mesophyll and not
their bundle shealth. They suffer from photorespiration on hot dry days. "C4
plants" have chloroplasts in both cells; the bundle shealth has RuBP, but the
mesophyll has a different binding molecule (PEP) with a higher affinity for
CO2. So, PEP (a C3 molecule) can bind CO2
at low concentrations, and then the product (a C4 product) is
passed to the bundle shealth. In the bundle shealth the CO2 is dissociated
from PEP, and PEP is returned to the mesophyll. This keeps the concentration
of CO2 in the bundle shealth high enough for RuBP to keep fixing
CO2, even though the leaf may be closed. So, PEP "pumps"
CO2 into the bundle shealth, keeping the concentration of CO2
high enough that photosynthesis (and not photorespiration) will occur. This
allows C4 plants to maintain glucose production even on hot dry days when their
stomates are closed. Grasses are classic C4 plants, and they have adapted
physiologically (and morphologically) to their environment.
"C3
plants" (as described above - called C3 because the first STABLE product of
carbon fixation is a C3 molecule) have chloroplasts in their mesophyll and not
their bundle shealth. They suffer from photorespiration on hot dry days. "C4
plants" have chloroplasts in both cells; the bundle shealth has RuBP, but the
mesophyll has a different binding molecule (PEP) with a higher affinity for
CO2. So, PEP (a C3 molecule) can bind CO2
at low concentrations, and then the product (a C4 product) is
passed to the bundle shealth. In the bundle shealth the CO2 is dissociated
from PEP, and PEP is returned to the mesophyll. This keeps the concentration
of CO2 in the bundle shealth high enough for RuBP to keep fixing
CO2, even though the leaf may be closed. So, PEP "pumps"
CO2 into the bundle shealth, keeping the concentration of CO2
high enough that photosynthesis (and not photorespiration) will occur. This
allows C4 plants to maintain glucose production even on hot dry days when their
stomates are closed. Grasses are classic C4 plants, and they have adapted
physiologically (and morphologically) to their environment.
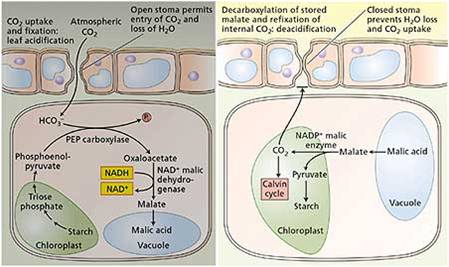
b. Crassulacean Acid Metabolism
(CAM)
"CAM plants" (for crassulacean
acid metabolism) fix CO2 at night and bind it to a C# molecule to
form malate (C4). Then, in the day when stomates are closed
but light is available, they harvest energy and split the malate to release
the CO2 to RUBP, allowing both reactions to proceed.
Study Questions:
1. Draw what happens
in the primitive light reaction of sulphur bacteria, and explain the events
that occur.
2. What is
the electron donor for sulphur bacteria? What type of limitation does this impose
on where these organisms can live?
 3.
Draw and explain what happens in the more advanced light dependent reaction.
Why can we call this an 'adaptation'? (Why is this an improvement over the the
more primitive system, considering the habitats available on Earth?)
3.
Draw and explain what happens in the more advanced light dependent reaction.
Why can we call this an 'adaptation'? (Why is this an improvement over the the
more primitive system, considering the habitats available on Earth?)
4. Describe
the correlations between these observations:
- the
oldest fossils are 3.8 billion years old and look like photosynthetic organisms
- eukaryotic
photosynthetic organisms about 2 billion years ago
- 'red
beds', the oldest sedimentary deposits that include oxidized minerals, date
to about 2 billion years
- previous
to these red beds, minerals in sedimentary deposits are in their reduced state,
suggesting that they were not exposed to an oxidizing atmosphere during their
erosion and deposition, suggesting that the atmosphere contained no oxygen
gas.
5. Draw the Light
Indepedent reaction and describe the events that occur.
6. Explain photorespiration
and describe two different adaptations of plants that live in hot, dry, environments.
7. When, where,
and why is oxygen produced by photosynthesis? What is the primary function
of photosynthesis?
 Photosynthesis
is a process of energy transformation. Again, although energy can neither be
created nor destroyed, it can be transformed. In the "Light Dependent Reaction"
radiant energy ('carried' by photons in light) is transformed into chemical
energy ('carried' by electrons). It requires an electron DONOR to provide electrons
that will 'carry' this energy. The energy 'carried' by this electron is used
to form a bond between ADP and P, creating ATP. Through this transfer, the electron
loses this energy. As we have discussed before, the phosphate bonds in ATP are
easily made and easily broken - that's why energy in this form of chemical bond
can be 'used' by all enzymes in the cell. However, ATP is readily hydrolyzed
in water...so it is difficult for a cell to build up a large amount of ATP before
it 'dissolves' to ADP and P again. To store large amounts of energy for a longer
time, the energy in ATP can be converted to a more stable molecule. In most
photosynthetic organisms, the catabolism of ATP is coupled to anabolic reactions
that bind carbon dioxide molecules together into stable molecules of glucose,
for longer term E storage. This also provides the cell with organic carbon that
it can use to make the other biologically important molecules. These are the
"Light Independent Reactions" of photosynthesis.
Photosynthesis
is a process of energy transformation. Again, although energy can neither be
created nor destroyed, it can be transformed. In the "Light Dependent Reaction"
radiant energy ('carried' by photons in light) is transformed into chemical
energy ('carried' by electrons). It requires an electron DONOR to provide electrons
that will 'carry' this energy. The energy 'carried' by this electron is used
to form a bond between ADP and P, creating ATP. Through this transfer, the electron
loses this energy. As we have discussed before, the phosphate bonds in ATP are
easily made and easily broken - that's why energy in this form of chemical bond
can be 'used' by all enzymes in the cell. However, ATP is readily hydrolyzed
in water...so it is difficult for a cell to build up a large amount of ATP before
it 'dissolves' to ADP and P again. To store large amounts of energy for a longer
time, the energy in ATP can be converted to a more stable molecule. In most
photosynthetic organisms, the catabolism of ATP is coupled to anabolic reactions
that bind carbon dioxide molecules together into stable molecules of glucose,
for longer term E storage. This also provides the cell with organic carbon that
it can use to make the other biologically important molecules. These are the
"Light Independent Reactions" of photosynthesis. When
we think of photosynthesis, most of us think "plants". This is generally
correct, but very incomplete. First, there are some plants like Indian Pipe
(Monotropa uniflora) that do not photosynthesize. Although they evolved
from photosynthetic ancestors, they have adopted a parasitic lifestyle and no
longer harvest their own energy from sunlight. In addition, there are photosynthetic
protists (algae and Euglenozoans), and photosynthetic archaeans and eubacteria.
In fact, there are several animals that harbor photosynthetic symbionts, too.
Many corals (corals are animals) ingest algal cells and distribute them to their
tentacles. The algae photosynthesize, and excess sugars are passed to the coral
animal. These symbiotic algae give corals their spectacular colors. When stressed
by water polution or high water temperatures, the corals release their symbionts
and lose their color ("a phenomenon called "coral bleaching").
Long periods without their symbionts results in coral death.
When
we think of photosynthesis, most of us think "plants". This is generally
correct, but very incomplete. First, there are some plants like Indian Pipe
(Monotropa uniflora) that do not photosynthesize. Although they evolved
from photosynthetic ancestors, they have adopted a parasitic lifestyle and no
longer harvest their own energy from sunlight. In addition, there are photosynthetic
protists (algae and Euglenozoans), and photosynthetic archaeans and eubacteria.
In fact, there are several animals that harbor photosynthetic symbionts, too.
Many corals (corals are animals) ingest algal cells and distribute them to their
tentacles. The algae photosynthesize, and excess sugars are passed to the coral
animal. These symbiotic algae give corals their spectacular colors. When stressed
by water polution or high water temperatures, the corals release their symbionts
and lose their color ("a phenomenon called "coral bleaching").
Long periods without their symbionts results in coral death.  Photosynthesis
in prokaryotes occurs on the double-membrane system of these organisms. In eukaryotes,
photosynthesis occurs in organelles called chloroplasts. Chloroplasts have a
bacteria-like double membrane, and they have their own DNA. This DNA is more
similar in most respects to the DNA in free-living bacteria than to the DNA
in the nucleus of the eukaryotic cells they 'inhabit'. For these reasons, most
scientists accept the 'endosymbiotic theory' of chloroplast origins. This theory
states that chloroplasts in the cells of photosynthetic eukaryotes are descendants
of free-living photosynthetic bacteria. At some point in the early evolution
of protists, these photosynthetic bacteria were engulfed by not digested. Rather,
the host cells fed on the excess sugars produced by the internalized bacteria.
Eventually, as the result of gene exchange between the host and proto-chloroplasts,
the eukaryotic host and the prokaryotic symbiont became dependent on one another.
But chloroplasts can still live outside of cells for several days. Plants, evolving
from green algae ancestors, inherited these bacteria-like chloroplasts, too.
Photosynthesis
in prokaryotes occurs on the double-membrane system of these organisms. In eukaryotes,
photosynthesis occurs in organelles called chloroplasts. Chloroplasts have a
bacteria-like double membrane, and they have their own DNA. This DNA is more
similar in most respects to the DNA in free-living bacteria than to the DNA
in the nucleus of the eukaryotic cells they 'inhabit'. For these reasons, most
scientists accept the 'endosymbiotic theory' of chloroplast origins. This theory
states that chloroplasts in the cells of photosynthetic eukaryotes are descendants
of free-living photosynthetic bacteria. At some point in the early evolution
of protists, these photosynthetic bacteria were engulfed by not digested. Rather,
the host cells fed on the excess sugars produced by the internalized bacteria.
Eventually, as the result of gene exchange between the host and proto-chloroplasts,
the eukaryotic host and the prokaryotic symbiont became dependent on one another.
But chloroplasts can still live outside of cells for several days. Plants, evolving
from green algae ancestors, inherited these bacteria-like chloroplasts, too. Photosynthesis
is a critically important process in the evolution and diversity of life. Prior
to the evolution of photosynthesis, life was dependent on absorbing spontaneously
generated organic molecules, or preying on other cells. Neither of these sources
of energy was probably all that common and easy to find. Evolving the ability
to use sunlight as an energy source, which IS abundant and IS easy to find,
meant that life could grow, prosper, and radiate dramatically - almost anywhere
there was a light source. Indeed, it looks like photosynthesis evolved very
early in the history of life; the earliest fossils (stromatolites and filamentous
microfossils dating to ~3.5 by) look very similar to photosynthetic bacteria
that are alive today. When photosynthetic organisms became abundant, they provided
a food supply for a wider variety of heterotrophic cells. Heterotrophs could
then live anywhere phototrophs lived; they were not limited to those rare places
where biological molecules were forming spontaneously. So, complex bacterial
food webs evolved. These early photosynthetic organisms used a primitive form
of photosynthesis that did not produce oxygen as a waste product. So, even though
they flourished for a billion years, no oxygen was added to the atmosphere.
About 2.0 billion years ago, a 'modern' type of photosynthesis evolved that
used water as the electron donor and produced oxygen gas as a waste product.
The production of oxygen gas transformed the oceans (precipitating iron), and
eventually changed the atmosphere, as well. Although oxygen was probably a highly
toxic gas at first (because it is so reactive), life eventually evolved to tolerate
it and then to USE it in oxidative respiration. The evolution of aerobic respiration
allowed for more energy to be harvested from the catabolism of complex organic
molecules, and may have allowed for the evolution of more energy-demanding eukaryotes
and multicellular organisms. As you know, almost all food webs are ultimately
dependent on the photosynthetic organisms at the base of the "food chain"
(hydrothermal vent communities are a possible exception). We use this energy
to stick amino acids together to make our proteins, etc. Even the gas and oil
that powers our industrial societies was initally stored as glucose produced
by photosynthesis. Coal, gas, and oil are just fossilized plants - and we "burn"
that energy millions of years after it was converted from sunlight. We are powering
our societies with sunlight that hit the Earth millions of years ago. But not
only are you (and every other heterotroph) energetically dependant on photosynthetic
organisms for food, you are also indebted to them for changing the planet and
stimulating the evolution of eukaryotic and multicellular life. In short, there
are few processes more important to the history and current function of living
systems (and our petroleum-based economy) than photosynthesis.
Photosynthesis
is a critically important process in the evolution and diversity of life. Prior
to the evolution of photosynthesis, life was dependent on absorbing spontaneously
generated organic molecules, or preying on other cells. Neither of these sources
of energy was probably all that common and easy to find. Evolving the ability
to use sunlight as an energy source, which IS abundant and IS easy to find,
meant that life could grow, prosper, and radiate dramatically - almost anywhere
there was a light source. Indeed, it looks like photosynthesis evolved very
early in the history of life; the earliest fossils (stromatolites and filamentous
microfossils dating to ~3.5 by) look very similar to photosynthetic bacteria
that are alive today. When photosynthetic organisms became abundant, they provided
a food supply for a wider variety of heterotrophic cells. Heterotrophs could
then live anywhere phototrophs lived; they were not limited to those rare places
where biological molecules were forming spontaneously. So, complex bacterial
food webs evolved. These early photosynthetic organisms used a primitive form
of photosynthesis that did not produce oxygen as a waste product. So, even though
they flourished for a billion years, no oxygen was added to the atmosphere.
About 2.0 billion years ago, a 'modern' type of photosynthesis evolved that
used water as the electron donor and produced oxygen gas as a waste product.
The production of oxygen gas transformed the oceans (precipitating iron), and
eventually changed the atmosphere, as well. Although oxygen was probably a highly
toxic gas at first (because it is so reactive), life eventually evolved to tolerate
it and then to USE it in oxidative respiration. The evolution of aerobic respiration
allowed for more energy to be harvested from the catabolism of complex organic
molecules, and may have allowed for the evolution of more energy-demanding eukaryotes
and multicellular organisms. As you know, almost all food webs are ultimately
dependent on the photosynthetic organisms at the base of the "food chain"
(hydrothermal vent communities are a possible exception). We use this energy
to stick amino acids together to make our proteins, etc. Even the gas and oil
that powers our industrial societies was initally stored as glucose produced
by photosynthesis. Coal, gas, and oil are just fossilized plants - and we "burn"
that energy millions of years after it was converted from sunlight. We are powering
our societies with sunlight that hit the Earth millions of years ago. But not
only are you (and every other heterotroph) energetically dependant on photosynthetic
organisms for food, you are also indebted to them for changing the planet and
stimulating the evolution of eukaryotic and multicellular life. In short, there
are few processes more important to the history and current function of living
systems (and our petroleum-based economy) than photosynthesis. a.
cyclic phosphorylation in "purple non-sulphur" and "green non-sulpher"
bacteria:Like all
bacteria, they have a double membrane (two bilayers). Proteins nested within
the inner membrane form "reaction centers (also called "photosystems")
and "electron transport chains" (ETC's) used in photosynthesis. This
inner membrane is often highly convoluted, increasing the surface area and the
number of reaction centers and ETC's that can be imbedded. Each reaction
center contains proteins arrayed around molecules of bacteriochlorophyll, which
contain atoms of Magnesium. In the
presence of light, the photons transfer energy to these electrons. The electrons
are raised to a higher energy state, lost from
the atom, and transferred to an 'electron acceptor molecule' in the inner membrane
of the bacterium, which transfers the electron the the electron transport chain.
When a high-energy electron
is transferred down the chain, protons
(H+) follow ('electrostatically') and are pumped across the inner membrane into
the intramembrane space. This build-up of H+ ions in the intermembrane space
creates an electrostatic charge differential across the membrane. There are
closed protein channels that, when opened, allow the H+ to flood through in
response to the charge gradient. This electric discharge energy is used by the
enzyme ATP-synthetase to add a phosphate group to ADP, making ATP. This is called
'chemiosmotic synthesis' or 'chemiosmosis'. So, what has happened is that the
passage of an electron -excited by light energy - has been used to 'pump protons'
into the intermembrane space, establishing an H+ ion charge gradient. The flow
of H+ ions through protein channels transforms this electric energy to chemical
bond energy in the form of a bond between ADP and P--> ATP. The
high-energy electron is then passed down the electron transport chain. and ATP
is produced. The electron, having lost its energy, can be recycled back to the
Mg atom. This cyclic production of ATP, powered by sunlight, is called cyclic
phosphorylation. As discussed below, these odd bacteria do not perform the light
independent pathways. In other words, they do not use the energy in ATP to make
glucose. This has two interesting consequences. First, it means they can't rely
on photosynthesis, alone, for energy harvest, because ATP isn't stable enough
to last over the course of an evening. So, they must also 'eat' - they are heterotrophs,
and can harvest energy from the food they ingest. The other consequence is discussed
below.
a.
cyclic phosphorylation in "purple non-sulphur" and "green non-sulpher"
bacteria:Like all
bacteria, they have a double membrane (two bilayers). Proteins nested within
the inner membrane form "reaction centers (also called "photosystems")
and "electron transport chains" (ETC's) used in photosynthesis. This
inner membrane is often highly convoluted, increasing the surface area and the
number of reaction centers and ETC's that can be imbedded. Each reaction
center contains proteins arrayed around molecules of bacteriochlorophyll, which
contain atoms of Magnesium. In the
presence of light, the photons transfer energy to these electrons. The electrons
are raised to a higher energy state, lost from
the atom, and transferred to an 'electron acceptor molecule' in the inner membrane
of the bacterium, which transfers the electron the the electron transport chain.
When a high-energy electron
is transferred down the chain, protons
(H+) follow ('electrostatically') and are pumped across the inner membrane into
the intramembrane space. This build-up of H+ ions in the intermembrane space
creates an electrostatic charge differential across the membrane. There are
closed protein channels that, when opened, allow the H+ to flood through in
response to the charge gradient. This electric discharge energy is used by the
enzyme ATP-synthetase to add a phosphate group to ADP, making ATP. This is called
'chemiosmotic synthesis' or 'chemiosmosis'. So, what has happened is that the
passage of an electron -excited by light energy - has been used to 'pump protons'
into the intermembrane space, establishing an H+ ion charge gradient. The flow
of H+ ions through protein channels transforms this electric energy to chemical
bond energy in the form of a bond between ADP and P--> ATP. The
high-energy electron is then passed down the electron transport chain. and ATP
is produced. The electron, having lost its energy, can be recycled back to the
Mg atom. This cyclic production of ATP, powered by sunlight, is called cyclic
phosphorylation. As discussed below, these odd bacteria do not perform the light
independent pathways. In other words, they do not use the energy in ATP to make
glucose. This has two interesting consequences. First, it means they can't rely
on photosynthesis, alone, for energy harvest, because ATP isn't stable enough
to last over the course of an evening. So, they must also 'eat' - they are heterotrophs,
and can harvest energy from the food they ingest. The other consequence is discussed
below.
 b.
"green sulphur" and "purple
sulphur" bacteria that
use sulphides as the electron donors:
b.
"green sulphur" and "purple
sulphur" bacteria that
use sulphides as the electron donors: - Here's how it works: Light strikes the phosystems nested in the inner membrane
(called the 'thylakoid' membrane in chloroplasts). An electron in each photosystem
is excited and lost from the Mg in the chlorophyll molecule. The electrons are
accepted by partcular electron acceptor molecules. The electron lost from PS
I is ultimately passed to NADP, which accepts a H+ to balance the charge, making
the high energy molecule, NADPH. The electron lost from PSII is passed to an
electron acceptor, and then to molecules in the electron transport chain. As
the electron is passed down the chain, ATP is produced by chemiosmosis (as described
above). When this electron has lost it's energy, it replaces the electron lost
from PS I. So, PS I is all set, and need not strip electrons from an electron
donor. However, PS II has lost an electron, and must replace this electron for
photosynthesis to continue. PSII strips electrons from H2O. Water
is split into oxygen, 2 H+, and 2 electrons. The electrons are passed to the
cholorophyll in PS II, excited by light, and energized. The oxygen reacts with
another oxygen atom to produce oxygen gas, which is released as a waste product.
The propose of photosynthesis is not "to produce oxygen". The purpose
of the light reaction of photosynthesis is to transform radiant energy into
chemcial energy, and produce ATP and NADPH. The two molecules, ATP and NADPH,
are the useful products. Again, oxygen gas is produced as a waste product when
electrons are stripped from water. The presence of oxygen in the oceans 2.5-2
billion years ago, indicated by the presence of sedimentary deposits with oxidized
iron (banded iron formations), indicates the evolution of this more advanced
type of photosynthesis that evolved in ancient photosynthetic bacteria.
- Here's how it works: Light strikes the phosystems nested in the inner membrane
(called the 'thylakoid' membrane in chloroplasts). An electron in each photosystem
is excited and lost from the Mg in the chlorophyll molecule. The electrons are
accepted by partcular electron acceptor molecules. The electron lost from PS
I is ultimately passed to NADP, which accepts a H+ to balance the charge, making
the high energy molecule, NADPH. The electron lost from PSII is passed to an
electron acceptor, and then to molecules in the electron transport chain. As
the electron is passed down the chain, ATP is produced by chemiosmosis (as described
above). When this electron has lost it's energy, it replaces the electron lost
from PS I. So, PS I is all set, and need not strip electrons from an electron
donor. However, PS II has lost an electron, and must replace this electron for
photosynthesis to continue. PSII strips electrons from H2O. Water
is split into oxygen, 2 H+, and 2 electrons. The electrons are passed to the
cholorophyll in PS II, excited by light, and energized. The oxygen reacts with
another oxygen atom to produce oxygen gas, which is released as a waste product.
The propose of photosynthesis is not "to produce oxygen". The purpose
of the light reaction of photosynthesis is to transform radiant energy into
chemcial energy, and produce ATP and NADPH. The two molecules, ATP and NADPH,
are the useful products. Again, oxygen gas is produced as a waste product when
electrons are stripped from water. The presence of oxygen in the oceans 2.5-2
billion years ago, indicated by the presence of sedimentary deposits with oxidized
iron (banded iron formations), indicates the evolution of this more advanced
type of photosynthesis that evolved in ancient photosynthetic bacteria.
 The
primary reaction is called the Calvin-Benson
Cycle, and it works like this:
The
primary reaction is called the Calvin-Benson
Cycle, and it works like this:  RuBP
will bind to BOTH CO2 and O2. And when RuBP binds
to O2, it is split and transformed to the amino acid serine, with
the production of CO2 as waste. Essentially, it is digested.
These reactions use the ATP and NADPH produced in the light reaction, too. So,
when RuBP binds O2, it does the exact opposite of photosynthesis
- it IS RESPIRED - the energy of the light reaction is used to BREAK DOWN a
carbohydrate (RuBP) and RELEASE CO2. This happens when the relative
concentrations of O2 and CO2 cross a critical threshold,
If O2 is super-abundant and CO2 is scarce, then photorespiration
will ocur. Oxygen levels can rise to these levels on HOT, DRY days, when there
is a lot of sun for photosynthesis to proceed (so O2 concentrations
rise in the leaf and CO2 concentrations fall), and it is DRY so the
stomates are closed and gases can't be exchanged with the environment (so O2
builds up in the leaf). It may seem curious that such a critical molecule has
such a debilitating characteristic. However, because
RuBP
will bind to BOTH CO2 and O2. And when RuBP binds
to O2, it is split and transformed to the amino acid serine, with
the production of CO2 as waste. Essentially, it is digested.
These reactions use the ATP and NADPH produced in the light reaction, too. So,
when RuBP binds O2, it does the exact opposite of photosynthesis
- it IS RESPIRED - the energy of the light reaction is used to BREAK DOWN a
carbohydrate (RuBP) and RELEASE CO2. This happens when the relative
concentrations of O2 and CO2 cross a critical threshold,
If O2 is super-abundant and CO2 is scarce, then photorespiration
will ocur. Oxygen levels can rise to these levels on HOT, DRY days, when there
is a lot of sun for photosynthesis to proceed (so O2 concentrations
rise in the leaf and CO2 concentrations fall), and it is DRY so the
stomates are closed and gases can't be exchanged with the environment (so O2
builds up in the leaf). It may seem curious that such a critical molecule has
such a debilitating characteristic. However, because  "C3
plants" (as described above - called C3 because the first STABLE product of
carbon fixation is a C3 molecule) have chloroplasts in their mesophyll and not
their bundle shealth. They suffer from photorespiration on hot dry days. "C4
plants" have chloroplasts in both cells; the bundle shealth has RuBP, but the
mesophyll has a different binding molecule (PEP) with a higher affinity for
CO2. So, PEP (a C3 molecule) can bind CO2
at low concentrations, and then the product (a C4 product) is
passed to the bundle shealth. In the bundle shealth the CO2 is dissociated
from PEP, and PEP is returned to the mesophyll. This keeps the concentration
of CO2 in the bundle shealth high enough for RuBP to keep fixing
CO2, even though the leaf may be closed. So, PEP "pumps"
CO2 into the bundle shealth, keeping the concentration of CO2
high enough that photosynthesis (and not photorespiration) will occur. This
allows C4 plants to maintain glucose production even on hot dry days when their
stomates are closed. Grasses are classic C4 plants, and they have adapted
physiologically (and morphologically) to their environment.
"C3
plants" (as described above - called C3 because the first STABLE product of
carbon fixation is a C3 molecule) have chloroplasts in their mesophyll and not
their bundle shealth. They suffer from photorespiration on hot dry days. "C4
plants" have chloroplasts in both cells; the bundle shealth has RuBP, but the
mesophyll has a different binding molecule (PEP) with a higher affinity for
CO2. So, PEP (a C3 molecule) can bind CO2
at low concentrations, and then the product (a C4 product) is
passed to the bundle shealth. In the bundle shealth the CO2 is dissociated
from PEP, and PEP is returned to the mesophyll. This keeps the concentration
of CO2 in the bundle shealth high enough for RuBP to keep fixing
CO2, even though the leaf may be closed. So, PEP "pumps"
CO2 into the bundle shealth, keeping the concentration of CO2
high enough that photosynthesis (and not photorespiration) will occur. This
allows C4 plants to maintain glucose production even on hot dry days when their
stomates are closed. Grasses are classic C4 plants, and they have adapted
physiologically (and morphologically) to their environment.
 3.
Draw and explain what happens in the more advanced light dependent reaction.
Why can we call this an 'adaptation'? (Why is this an improvement over the the
more primitive system, considering the habitats available on Earth?)
3.
Draw and explain what happens in the more advanced light dependent reaction.
Why can we call this an 'adaptation'? (Why is this an improvement over the the
more primitive system, considering the habitats available on Earth?)