 A. Structure
A. Structure
I. Carbohydrates
 A. Structure
A. Structure
- monomer - monosaccharide (simple sugar) - CnH2nOn
(glucose, galactose, fructose are 6 carbon sugars; ribose, ribulose, deoxyribose
are 5 carbon sugars)
- disaccharides: sucrose (glucose + fructose); maltose - (2 glucose)
- polymer - polysaccharide - chain of sugars
starch, glycogen, chitin, cellulose
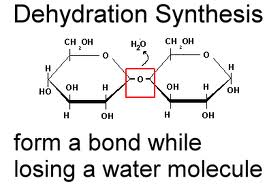 - monomers are linked together into polymers using dehydration synthesis - a
removal of a water molecule (dehydration) and the synthesis of a bond. This
requires energy and is catalyzed by enzymes in living systems.
- monomers are linked together into polymers using dehydration synthesis - a
removal of a water molecule (dehydration) and the synthesis of a bond. This
requires energy and is catalyzed by enzymes in living systems.
B. Function:
1. energy storage:
- all large biomolecules have lots of bonds and thus store lots of energy.
But, the larger the molecule, the more time it takes to harvest all the energy
by metabolic breakdown (catabolism). So, polysaccarides serve better as
'longer-term' energy storage than monosaccharides, whereas monosaccarides, because
they can be metabolized more quickly, serve better as a short term energy supply.
(starch in plants andglycogen in the liver of animals are longer term storage
molecules; glucose is the short-term energy molecule in all of life)
2. Structural:
- cellulose is just a long chain of glucose. And decomposers break down
wood to create the sugars they will use for metabolic energy.
- chitin is the primary component of exoskeletons in arthropods.
II. Proteins
 - (we
will cover this in more detail during the lecture on protein synthesis)
- (we
will cover this in more detail during the lecture on protein synthesis)
A. Structure:
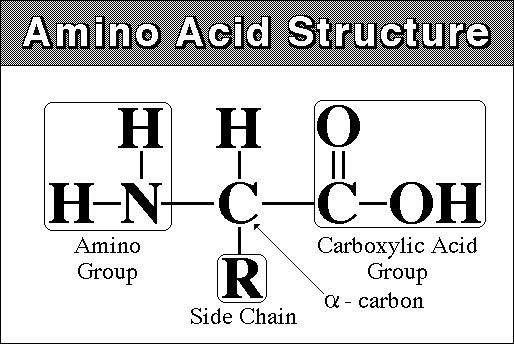
monomer - amino acid - amine (NH2) group at one end and carboxyl
group (COOH) at other
there are 20 different amino acids that are found in living systems.
polymer - polypeptide - 100 to 300 amino acids long. The AA's are linked by dehydration synthesis reactions into a long linear chain. Because there are 20 Amino Acids ("letters") that can be used in their construction, proteins can have a limitless number of different combinations (like letters in different combinations make differnt "words"). This variety in form means variety in function.
**Higher levels of structure:
1. the primary structure of a polypeptide/protein is the linear sequence of amino acids
2. this linear sequence can take a helical or "pleated" sheet shape, depending on bond angles and soforth. These are secondary levels structure
3. some proteins then fold upon themselves, taking a globular shape. This globular shape is maintained by bonds between different functional groups of differnt amino acids. Enzymes and cell membrane proteins are common globular proteins.this is called tertiary structure.
4. Sometimes, single proteins are not functional on their own - they must be combined with other proteins to forma a protein with a quaternary structure. Hemoglobin, with 2 alpha and 2 beta globular polypeptides, is one example. collagen is another, composed of several helical polypeptides.
B. Function:
1. Energy Storage: (all biomolecules
can be broken down for energy harvest. Typically, since proteins are doing
something else, too, they are broken down last so that the organism can maintain
this function that the protein performs for as long as possible).
2. Structural:
after water, animals are largely proteinaceous
collagen, elastin, muscle proteins, etc.
3. Metabolic:
all biological reactions are catalyzed. Most biological catalysts are
proteinaceous ENZYMES
4. transport:
cell membrane - there are proteins that assist transport across the membrane
organism - hemoglobin, for instance, transport oxygen
5. Immunity:
antibodies are proteins.
III. Fats and Lipids
A. Structure:
monomer -
fatty acid - long carbon chain with a carboxyl group (COOH)
- can be saturated (with H - no double bonds between C's) or unsaturated (a
double bond)
- animal fats are usually saturated, and are solid at room temp. Plant and fish
fats are usually unsaturated, and are liquid at room temp and are called 'oils'.
By saturating a plant fat, it can be made solid - hydrogenated fat or oil. (changing
peanut oil into peanut butter, or vegetable oil into "crisco"). During
this process, trans-fats are also created. These are unsaturated fats with a
trans (not cis) conformation. Trans-fats have been associated with atherosclerosis
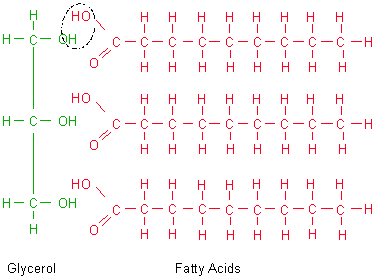 polymer - fat (triglyceride)
polymer - fat (triglyceride)
- three fatty acids attached by dehydration synthesis to a glycerol molecule
- phospholipid: glycerol with 2 fatty acids and a phosphate (PO4) group.
The PO4 is negatively charged (thus, polar), while the fatty acids are non-polar.
This accounts for how these molecules orient in aqueous solutions, forming membranes.
A "choline" groups is typically attached to the phosphate.
B. Function:
1. Energy storage: saturated fats
are very dense - the fatty acids fit together, in parallel, very snugly.
So, to store the most energy in the smallest space, fats are the preferred medium.
2. Cell membranes - barrier to water soluble materials. The non-polar lipid "bilayer" is a barrier to water soluble materials (that are ionic or polar). So, ionic and polar compounds can't just flow into the cell; the cell can regulate how much of what gets in and out.
3. Insulation
4. Hormones - derived from fats,
lipid soluble, slip right theough cell membranes into cells, so they can function
at very low concentrations.
Study Questions:
1. What is the basic structure of a monosaccharide?
2. What type of molecule are chitin and cellulose?
3. Show how two monosaccharides are linked together. What is the name of this reaction?
4. What is the structure of a fatty acid? A triglyceride?
5. What is the monomeric unit of proteins? Draw one, without specifying the variable group.
6. Show how two amino acids are linked together. What is the name of the reaction?