 C. Proteins - (we
will cover this in more detil during the lecture on protein synthesis)
C. Proteins - (we
will cover this in more detil during the lecture on protein synthesis)
 C. Proteins - (we
will cover this in more detil during the lecture on protein synthesis)
C. Proteins - (we
will cover this in more detil during the lecture on protein synthesis)
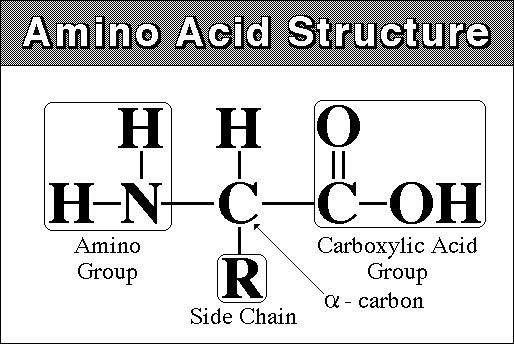
1. Structure:
monomer - amino acid - amine (NH2) group at one end and carboxyl
group (COOH) at other
there are 20 different amino acids that are found in living systems.
polymer - polypeptide - 100 to 300 amino acids long. The AA's are linked by dehydration synthesis reactions into a long linear chain. Because there are 20 Amino Acids ("letters") that can be used in their construction, proteins can have a limitless number of different combinations (like letters in different combinations make differnt "words"). This variety in form means variety in function.
**Higher levels of structure:
1. the primary structure of a polypeptide/protein is the linear sequence of amino acids
2. this linear sequence can take a helical or "pleated" sheet shape, depending on bond angles and soforth. These are secondary levels structure
3. some proteins then fold upon themselves, taking a globular shape. This globular shape is maintained by bonds between different functional groups of differnt amino acids. Enzymes and cell membrane proteins are common globular proteins.this is called tertiary structure.
4. Sometimes, single proteins are not functional on their own - they must be combined with other proteins to forma a protein with a quaternary structure. Hemoglobin, with 2 alpha and 2 beta globular polypeptides, is one example. collagen is another, composed of several helical polypeptides.
2. Function:
a. Energy Storage: (all biomolecules
can be broken down for energy harvest. Typically, since proteins are doing
something else, too, they are broken down last so that the organism can maintain
this function that the protein performs for as long as possible).
b. Structural:
after water, animals are largely proteinaceous
collagen, elastin, muscle proteins, etc.
c. Metabolic:
all biological reactions are catalyzed. Most biological catalysts are
proteinaceous ENZYMES
d. transport:
cell membrane - there are proteins that assist transport across the membrane
organism - hemoglobin, for instance, transport oxygen
e. Immunity:
antibodies are proteins.
1. Structure:
monomer -
fatty acid - long carbon chain with a carboxyl group (COOH)
- can be saturated (with H - no double bonds between C's) or unsaturated (a
double bond)
- animal fats are usually saturated, and are solid at room temp. Plant and fish
fats are usually unsaturated, and are liquid at room temp and are called 'oils'.
By saturating a plant fat, it can be made solid - hydrogenated fat or oil. (changing
peanut oil into peanut butter, or vegetable oil into "crisco"). During
this process, trans-fats are also created. These are unsaturated fats with a
trans (not cis) conformation. Trans-fats have been associated with atherosclerosis
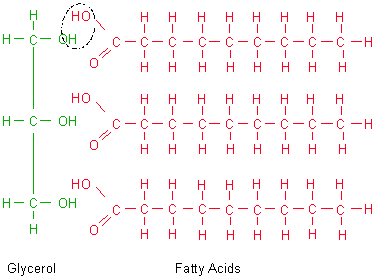 polymer - fat (triglyceride)
polymer - fat (triglyceride)
- three fatty acids attached by dehydration synthesis to a glycerol molecule
- phospholipid: glycerol with 2 fatty acids and a phosphate (PO4) group.
The PO4 is negatively charged (thus, polar), while the fatty acids are non-polar.
This accounts for how these molecules orient in aqueous solutions, forming membranes.
A "choline" groups is typically attached to the phosphate.
2. Function:
a. Energy storage: saturated fats
are very dense - the fatty acids fit together, in parallel, very snugly.
So, to store the most energy in the smallest space, fats are the preferred medium.
b. Cell membranes - barrier to water soluble materials. The non-polar lipid "bilayer" is a barrier to water soluble materials (that are ionic or polar). So, ionic and polar compounds can't just flow into the cell; the cell can regulate how much of what gets in and out.
c. Insulation
d. Hormones - derived from fats,
lipid soluble, slip right theough cell membranes into cells, so they can function
at very low concentrations.
DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) are nucleic acids - polymers consisting of a linear sequence of linked nucleotide monomers. We will describe the structure of the monomers first, and then describe how they are linked into linear polymers. Finally, we will describe the double-stranded structure of ds-DNA.
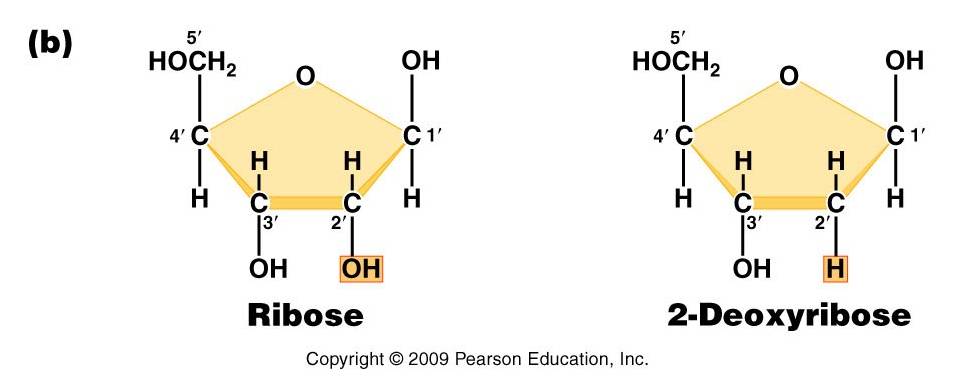
1. The monomers are "nucleotides"
three components:
- Pentose (5 carbon) sugar: either ribose (RNA) or deoxyribose (DNA). The carbons are numbered clockwise. The difference between the sugars is that ribose has an -OH group on the 2' carbon, whereas deoxyriboes has only 2 H groups and thus is "deoxygenated" relative to ribose. BOTH sugars have an -OH group on the 3' carbon, which will be involved in binding. The 5' carbon is a sidegroup off the ring.
- Nitrogenous Base: each nucleotide has a single nitrogenous base attached to the 1' carbon of the sugar. This nitrogenous base may be a double-ringed structure (purine) or a single ringed (pyrimidine) structure. The purines are adenine (A) and guanine (G). The pyrimidines are thymine (T), cytosine (C), and uracil (U). DNA nucleotides may carry A, G, C, or T. RNA nucleotides carry either A, G, C, or U.
- The third component of a nucleotide is a phosphate group, which is attached to the 5' carbon of the sugar. When a nucleotide is incorporated into a chain, it has a single phosphate group. However, nucleotides can occur that have two or three phosphate groups (dinucleotides and trinucleotides). ADP and ATP are important examples of these types of molecules. In fact, the precursors of incorporated nucleotides are trinucleotides. When two phosphates are cleaved, energy is released that can be used to add the remaining monophosphate nucleotide to the nucleic acid chain.
2. Polymerization is by 'dehydration synthesis'
As with all other classes of biologically important polymers, monomers are linked into polymers by dehydration synthesis. In nucleic acid formation, this involves binding the phosphate group of one nucleotide to the -OH group on the 3' carbon of the existing chain. For the purposes of seeing how this reaction works, we can envision an H+ on one of the negatively charged oxygens of the phosphate group. Then, a molceule of water can be removed from these two -OH groups, leaving an oxygen binding the sugar of one nucleotide to the phosphate of the next.
This creates a 'dinucleotide'. It has a polarity/directionality; it is different at its ends. At one end, the reactive group is the phosophate on the 5' carbon. This is called the 5' end of the chain. At the other end, the reactive group is the free -OH on the 3' carbon; this is the 3' end of the chain. So, a nucleic acid strand has a 5' - 3' polarity.
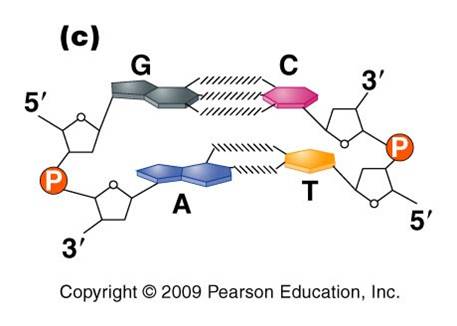 3.
Most DNA exists as a 'double helix' (ds-DNA) containing two linear nucleic acid
chains.
3.
Most DNA exists as a 'double helix' (ds-DNA) containing two linear nucleic acid
chains.
a. the nitrogenous bases on the two strands are 'complementary' to each other, and form weak hydrogen bonds between them. A always pairs with T, and C always pairs with G. As such, there is always a double-ringed purine pairing with a single-ringed pyrimidine, and the width of the double-helix is constant over its entire length.
b. the two strands (helices) are anti-parallel: they are arranged with opposite polarity. One strands points 5' - 3', while the other points 3' - 5'. The direction of the pentose sugars and the type of reactive group at the ends of the chains show this relationship.
4. RNA performs a wide variety of functions in living cells:
a. m-RNA (for "messenger") is the copy of a gene. It is the sequence of nitrogenous bases in m-RNA that is actually read by the ribosome to determine the structure of a protein.
b. r-RNA (for "ribosomal") is made the same way, as a copy of DNA. However, it is not carrying the recipe for a protein; rather, it is functional as RNA. It is placed IN the Ribosome, and it helps to ‘read’ the m-RNA.
c. t-RNA (for "transfer") is also made as a copy of DNA, but it is also functional as an RNA molecule. Its function is to bind to a specific amino acid and incorporate it into the amino acid sequence as instructed by the m-RNA and ribosome.
d. mi-RNA (micro-RNA) and si-RNA (small interfering RNA) bind to m-RNA and splice it; inhibiting the synthesis of its protein. This is a regulatory function.
e. sn-RNA (small nuclear RNA) are short sequences that process initial m-RNA products, and also regulate the production of r-RNA, maintain telomeres, and regulate the action of transcription factors. Regulatory functions.
Cell biology is another great thread of biology. Evolution explains life's diversity, genetics and heredity explain how life reproduces itself, and cell biology explains the fundamental structure of living systems. The birth of cell biology can be traced to the observations of Robert Hooke in 1655, who observed thin sections of cork through a microscope. The box-like compartments reminded him of cells in a monastery, and he coined the term. Of course, he was not observing true living cells - only the cell walls in dead plant tissue. The first observation of live cells was made by Antony van Leeuwenhoek in 1674 who observed protists, algal cells, and bacteria. In 1838-9, Schleiden and Schwann independently proposed that all plants and animals (respectively) were composed of cells - the 'cell theory' of life. They also supported the notion that cells propogated 'spontaneously' like crystals; but this would premise would be rejected by Virchow who, in 1858, famously wrote "omnis cellula e cellula" - 'all cells from cells'. Several cellular structures were observed during this period, as well: Brown described the nucleus in 1833, Kolliker described mitochondria in 1857, and Flemming described the movement of chromosomes and the stages of mitosis in 1879. Robert Koch isolated the bacteria that caused anthrax (1876), TB (1882), and cholera (1883). Pasteur developed vaccines and treatments for killing bacteria and fungi with heat (pasteurization). The contributions of Koch and Pasteur firmly established the 'germ theory' of disease and finally laid the idea of spontaneous generation - even by microbes - to rest. Following Van Beneden's description of the movement of chromosomes during meiosis in 1883, August Weismann proposed that the 'germ cells' of egg and sperm, although produced by 'somatic' body cells, were separated from them and were uninfluenced by them (1893); eliminating the possibility of the inheritance of characteristics acquired by the body. After the 'rediscovery' of Mendel's work in 1900 by Tschermak, DeVries, and Correns, Walter Sutton (1902) unified heredity and cell biology by recognizing the correlation between the movement of chromosomes during meiosis and Mendel's principles of heredity. He proposed that chromosomes carry the hereditary material - the 'chromosomal theory of heredity'.
All living things are composed of cells, so understanding how a cell works is fundamental to understanding all living systems (reductionism, remember?). Before we examine the patterns of heredity and the structure and function of DNA (topics directly relevant to our discussion of evolution), we will quickly review the cellular context in which DNA functions. At the most simplistic level, cells must absorb matter/energy, convert this matter/energy into a useable form, and then use this energy and matter to make the things they need, respond to the environment, and reproduce. To understand the material that follows, you must know the basics of atom structure, types of chemical bonds, the structure and properties of water, and the basic structure of biologically important molecules. This is basic science stuff covered in every high school chemistry and biology class, so it is assume knowledge here. If you want an overview of what you are responsible for, see this link: review of atoms, bonds, and molecules.
- All truly living things are composed of cells - from bacteria to blue whales to sequioas. Viruses, which are not capable of independendent metabolism and reproduction, are not cellular and straddle the definition of 'living'. And surprizingly (in some respects), these cells are all remarkably similar in their basic structure and function. They all have a plasma membrane composed of a lipid bilayer. They all have genetic material composed of DNA, and protein synthesis is performed by ribosomes. In addition, all cells can metabolize glucose and use ATP as the 'energy currency' in the cell.
- Although the largest bacterium so far discovered (Thiomargarita namibiensis) is 0.7mm (millimeters) long, most prokaryotic cells are thousands of time smaller, typically 0.2 - 2.0 um (micrometers)! And most prokaryotic cells are typically 100 times smaller than typical eukaryotic cells (usually 10-100 um). The largest eukaryotic cell, by weight, is an unfertilized ostrich egg. The longest eukaryotic cell may be a neuron in the leg of a giraffe, with the cell body in the spinal cord and the axon running the entire length of the leg.
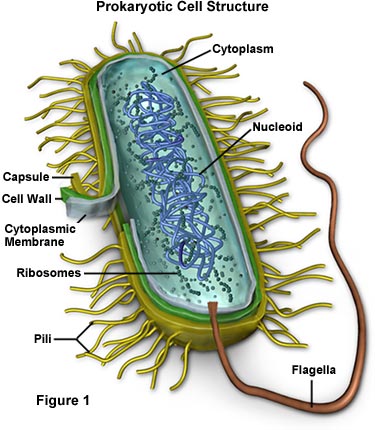 1.
Prokaryotic Cells
1.
Prokaryotic Cells
Prokaryotes ('eubacteria' and 'archaea') have cells that lack a nuclear membrane and membrane-bound organelles. Many have a polysaccharide coating (glycocalyx), or a cell wall. They also lack a cytoskeleton, and their flagella are structurally simple. They divide by binary fission - a much simpler process than the more complicated mitotic division that eukaryotes perform. And finally, prokaryotes do not produce specialized haploid gametes for sexual reproduction. However, they can exchange DNA between cells to create new genetic combinations. Although we often think of bacterial cells as independent organisms, most bacteria live in complex aggregates called 'biofilms'. When the cells join a biofilm, their physiology changes and they become a contributing member of a larger complex colony of microbial life nested in a protective layer of the slime they secrete. Examples of biofilms are dental plaque, the slime inside water and sewer pipes, and large colonies of bacterial and algal mats called stromatolites. As we will see later, although bacteria are not that structurally diverse (rods, spheres, and spirals), they are in many ways more metabolically diverse than eukaryotic cells.
2. Eukaryotic Cells
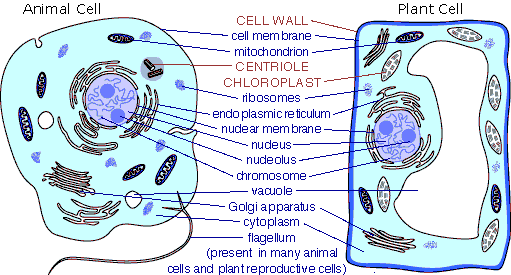 Eukaryotes
(protists, plants, fungi, and animals) have cells with a nucleus at some stage
in their life cycle (some cells like red blood cells may lose this nuclear membrane).
All eukaryotes except a few unusual protists have mitochondria - internal, cell-like
structures with their own membranes and DNA. The photosynthetic eukaryotes also
have membrane-bound chloroplasts. Their ribosomes are larger than those of prokaryotes,
but they perform the same function of protein assembly. They also have an internal
membrane system of the Endoplasmic Reticulum, Golgi Apparati, and Liposomes.
Chromosomes are circular in some protists, and are linear in other eukaryotes.
Some plants have more than 1000 chromosomes
in the nucleus of each cell. Cells of plants, fungi, and some protists
have protective cell walls composed of cellulose (plants and some protists)
or chitin (fungi and some protists). Plant cells also have a large central vacuole
for water storage and water regulation. Vacuoles and vesicles are compartments
that occur in the cells of other life forms, too - for the storage of nutrients
or waste.
Eukaryotes
(protists, plants, fungi, and animals) have cells with a nucleus at some stage
in their life cycle (some cells like red blood cells may lose this nuclear membrane).
All eukaryotes except a few unusual protists have mitochondria - internal, cell-like
structures with their own membranes and DNA. The photosynthetic eukaryotes also
have membrane-bound chloroplasts. Their ribosomes are larger than those of prokaryotes,
but they perform the same function of protein assembly. They also have an internal
membrane system of the Endoplasmic Reticulum, Golgi Apparati, and Liposomes.
Chromosomes are circular in some protists, and are linear in other eukaryotes.
Some plants have more than 1000 chromosomes
in the nucleus of each cell. Cells of plants, fungi, and some protists
have protective cell walls composed of cellulose (plants and some protists)
or chitin (fungi and some protists). Plant cells also have a large central vacuole
for water storage and water regulation. Vacuoles and vesicles are compartments
that occur in the cells of other life forms, too - for the storage of nutrients
or waste.
Consider a new cell just produced by binary fission or mitotic division of a pre-existing cell. This cell is roughly half the size of the parental cell. To reach the size of the parental cell, it must grow - this involves the synthesis of more membranes, more ribosomes, and more of all the other components of a cell. In addition, some molecules are breaking down naturally and must be replaced or repaired. So, a cell has to make stuff to keep itself alive and grow. The first law of thermodynamics states that: "energy/matter can not created or destroyed, only transformed to other types of matter/energy". So, for a cell to maintain itself and grow, it can't just 'create' this new matter of membranes and ribosomes from nothing; it must take in energy and matter and transform that energy/matter into these necessary structures. Cells harvest energy and create new molecules through chemical reactions - breaking molecules apart and linking their pieces together in new combinations. These chemical reactions are catalyzed by enzymes. The enzymes are molecules made by the cell, too, through other enzymatically catalyzed chemical reactions. Most enzymes are proteins. So, to make the lipids, polysaccharides, proteins, and nucleic acids that a cell needs, it must make the proteinaceous enzymes needed to sythesize these molecules. Protein (enzyme) synthesis is thus fundamental to everything else a cell does.
We can summarize cell function like this (for eukaryotic cells): biological molecules are absorbed across the membrane. Through chemical reactions (catalyzed by enzymes) in the cytoplasm and the mitochondria, the covalent bonds in these organic molecules are broken and some of the energy released by the breaking of these bonds is used to add a phosphate group to ADP--> making ATP. This is cellular respiration - transforming the energy contained in the covalent bonds of diverse organic molecules into the energy in a weak covalent bond of a single type of organic molecule (ATP). All enzymes can break these weak bonds in ATP to catalyze their reactions. Photosynthetic cells can also use the energy in sunlight to link a phosphate group to ADP--> making ATP; converting radiant energy into chemical energy of a covalent bond. In these two ways, cell transform energy in a diverse range of nutrients or light into one form of chemical of energy (bonds in ATP) that can be used throughout the cell. Much of this energy is used to link amino acids together to make proteins. These reactions occur at the ribosomes, which many be free in the cytoplasm or bound to the endoplasmic reticulum (ER), making the ER appear fuzzy or 'rough'. Proteins synthesized by ribosomes on the rough ER are shunted into the lumen (tube) of the ER, where they are passed to the Golgi Apparatus for processing (changing the initial protein product into a functional protein by cutting off some amino acids, and or binding it to another protein, fat, or carbohydrate). Liposomes budding off from the Golgi transfer the final protein product to the cell membrane, for incorporation in the membrane or delivery outside the cell. The sequence of amino acids in each protein is determined by the linear sequence of nitrogenous bases in genes - regions of DNA in chromosomes. The DNA double helix is opened, and the sequence of A,T, C, and G's is read. Based on this sequence, a complementary sequence of RNA is synthesized. This RNA molecule - essentially a copy of the gene - is shunted outside the nucleus to the ribosome. It is this RNA which is "read" by the ribosome, and determines the particular sequence of amino acids linked together into the initial protein product. All of these steps, from uncoiling the DNA, to reading the DNA, to making the RNA, to moving the RNA to the ribosome, the linking amino acids together to form the protein require other specific enzymes and energy in the form of ATP. Throgh this process of protein synthesis, the cell makes the enzymes it needs to synthesize new structural and transport proteins, new phospholipids, new polysaccharides, and new chromsomes. Once new chromosomes are synthesized, the cell can divide into two new cells. That's a very simple look at how cells live. It involves absorbing matter/energy, converting that energy to a usable form, making proteins based on the recipes in the DNA, and then reproducing. We will now look at these steps in more detail.
All living cells are bounded by a membrane composed of a phosopholipid bilayer. Proteins are present on and within each layer, and some also cross all the way through the membrane.
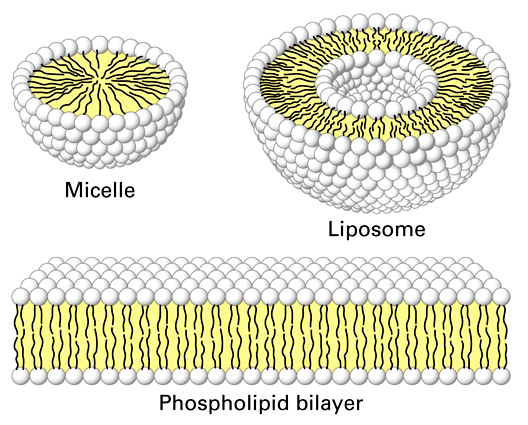 1.
Phospholipid Bilayer:
1.
Phospholipid Bilayer:
- phospholipids have hydrophobic
fatty acid tails and hydrophilic phosphate group "heads" (charged).
In an aqueous solution, phospholipids will form micelles (single layer spheres)
and bilayers (two layer spheres and films) as a function of the hydrophobic
and hydrophilic nature of these molecules. These spatial orientations surround
the hydrophobic fatty acid 'tails' with the hydrophilic phosphate 'heads' that
interact with the polar water molecules in the solution. When arranged as a
bilayer, they separate the internal aqueous solution of the cell from the external
aqueous environment. Because the principle boundary is the hydrophobic, non-polar
layers of fatty acids, the bilayer is permeable to lipid-soluble materials but
not water soluble (polar and ionic) materials unless they are very small. These
bilayers are also very dynamic or "fluid"... the phospholipids are
moving laterally all the time, like the lipids in a soap bubble.
2. Proteins arepresent on both
inner and outer surfaces, and also extending through the lipid bilayer.
3. Carbohydrates on the surface;
usually attached to proteins and forming a "glycoprotein"
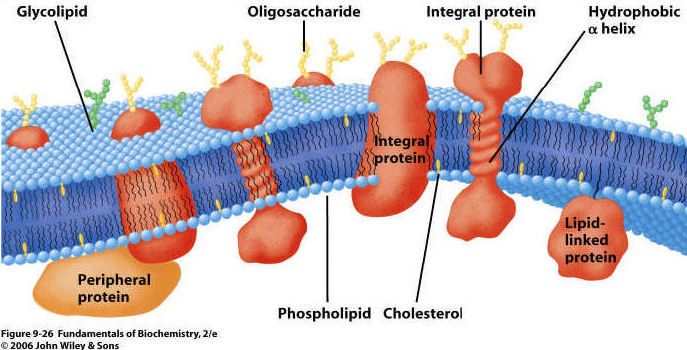
2. Transport: matter does cross the membrane. However, unless it is a small or non-polar molecule, it cannot cross the lipid bilayer "on its own". Rather, crossing the membrane must be assisted by a protein. These proteins may be rather unselective "tubes", or they may be specific for the transport of a particular class of molecules. By using proteins as 'gates' the cell gains control over the composition of its cytoplasm. There are three ways that material can cross a membrane:
a. Diffusion: As a consequence of the random movement of molecules, molecules will disperse from areas of high concentration and move to areas of lower concentration. So, when you uncork a bottle of perfume, the molecules diffuse through the room from high concnetration at the bottle to low concentration in the rest of the room. Molecules may cross membranes as they move in this manner, moving from areas of high concentration on one side of the membrane to areas of lower concentration on the other. With respect to the lipid bilayer in living membranes, non-polar molecules and some small molecules can diffuse directly through the lipid bilayer. The movement of CO2 and O2 across the membrane happens by diffusion. Every molecule moves by diffusion in response to its own concentration gradient.
The movement of water across a membrane, in response to its water potential, is called osmosis (video). Although water is polar, it is also a small molecule. So, it can cross the lipid bilayer directly, or through specialized protein channels called 'aquaporins'. Water potential is a bit more complicated than just 'concentration', although it includes this idea. The higher the concentration of dissolved solutes, the lower the 'concentration' of water and the lower the water potential. These solutes may be one or many things, so water potential is a function of total solute concentration. So, water will osmose across a membrane from an area of low solute concentration (high water potential) to an area of high solute concentration (low water potential), or in other words, from a dilute solution to a concentrated solution. Another component of water potential is water pressure: water will also cross a membrane due to pressure exerted by an outside force or the force of its own mass responding to gravity. So water can be pushed across a membrane.
Consider the figure, below. The purple circles are dissolved solutes. SO! In the first figure at left, total solute concentration is HIGH on the right side of the u-tube, so water potential is LOW . Water will move across the membrane from the dilute solution on the left (high water potential) to the concentrated solution on the right (low water potential), and the water level will rise (figure on the right). Eventually, in this rigid vertical system, the tendancy of the water to move left to right in response to solute concentration is balanced by the tendancy of water to move right to left - "leaking" back across the membrane - in response to the greater water pressure. An equilibrium is reached where there is no NET flow. In a rigid plant cell (with a cell wall), the influx of water creates "hydrostatic pressure" and makes the cell rigid or 'turgid'. The loss of water (from evapotranspiration or osmosis out of the cells to a saltier environment) reduces this turgidity - or 'turgor pressure' - and the plant tissue wilts (gets 'floppy'). In an animal cell bounded only by a thin membrane, the influx of water can create a pressure large enough to rupture the cell. We will deal with these concepts more in lab.
b. Faciliated Diffusion: Large polar molecules cannot diffuse across the lipid bilayer. However, they can cross the membrane 'passively' from high to low concentration, through integrated proteins channels. Some of these channels are rather unspecialized 'tubes', while other channels are rather specific and will only permit the transport of certain classes of molecules. In any case, this is the way that large polar molecules cross the membrane in response to their concentration gradient (high to low). video.
c. Active Transport: If material only crossed the membrane by some form of diffusion, then the cell's cytoplasm would come to be very similar to the surrounding environment. They cell would be unable to raise or lower the concentration of material above the concentration in the environment. In order for cells to be differnt from the environment (in the concetration of some stuff), another mechanism is needed. This is 'active transport'. In active transport, a cell uses energy to 'pump' material across the membrane - against the concentration gradient (from low to high). So, although sugars might be in higher concetration within the cell than outside it, and although sugars may be "leaking" from the cell by diffusion, the cell can pump sugar against the concentration gradient and accumulate it in the cell. Likewise, a cell can pump toxins or waste OUT of the cell, even if the concentration outside of the cell is already high. THIS TAKES ENERGY - LIKE ROLLING A BALL UPHILL, AGAINST THE 'GRADIENT' OR SLOPE. An important active transport mechanism is the "sodium-potassium" pump. In this process, both ions are pumped against their concentration gradient, using energy (breaking ATP) to change the conformation of the transport protein. video
3. Metabolism: Some of the proteins associated with the membrane are enzymes that catalyze reactions. By positioning enzymes next to one another that catalyze sequential reactions in a process, the process can run much more efficiently.
4. Signal transduction: Proteins can also be involved with 'perception'. When a membrane protein binds a compound in the environment, it may change shape. This shape change may release a subunit inside the cell that binds to something else and initiates a cellular response. So the cell perceives a stimulus in the environment and responds - all at the chemical level. The percetion of a signal can stimulate the activation or inactivation of a gene - and thus affect the proteins that a cell produces and the cell's basic physiology. video
5. Cell-cell recognition: Surface proteins and carbohydrates give a cell a chemical 'signature'. This is critical in the immune system, where cells are identified as "self" or "foreign" based on these surface antigens.
6. Cell binding: In many tissues (but not all, such as blood), the cells are bound together; sometimes quite tightly. This cell-cell binding usually involves proteins that interlock and bind together.
7. Attachment of the cytoskeleton: a cell is not a "baggie" with organelles floating around in the cytoplasm. Most organelles (like mitochondria and chromosomes) are bound to cytoskeletal fibers that hold them in position. The cytoskeleton is also responsible for changin the cells' shape.
 All living cells - eubacteria, archaea, protists, fungi, plants, and animals
- can harvest the energy contained in the chemical bonds of complex organic
molecules. By breaking the covalent bonds between carbon atoms in these molecules,
energy is released. The energy released by these catabolic reactions is used
to bind ADP + P --> ATP in coupled, anabolic reactions. As such, some of
the energy in the covalent bonds of the initial organic molecules is transformed
into chemical energy in bonds of ATP. Energy in this form is now available to
all of the enzymes in the cell, for catalyzing their own reactions (chemical
energy) or doing work like muscular contraction (mechanical energy) or pumping
ions across a membrane against their concentration gradient (active transport).
All living cells - eubacteria, archaea, protists, fungi, plants, and animals
- can harvest the energy contained in the chemical bonds of complex organic
molecules. By breaking the covalent bonds between carbon atoms in these molecules,
energy is released. The energy released by these catabolic reactions is used
to bind ADP + P --> ATP in coupled, anabolic reactions. As such, some of
the energy in the covalent bonds of the initial organic molecules is transformed
into chemical energy in bonds of ATP. Energy in this form is now available to
all of the enzymes in the cell, for catalyzing their own reactions (chemical
energy) or doing work like muscular contraction (mechanical energy) or pumping
ions across a membrane against their concentration gradient (active transport).
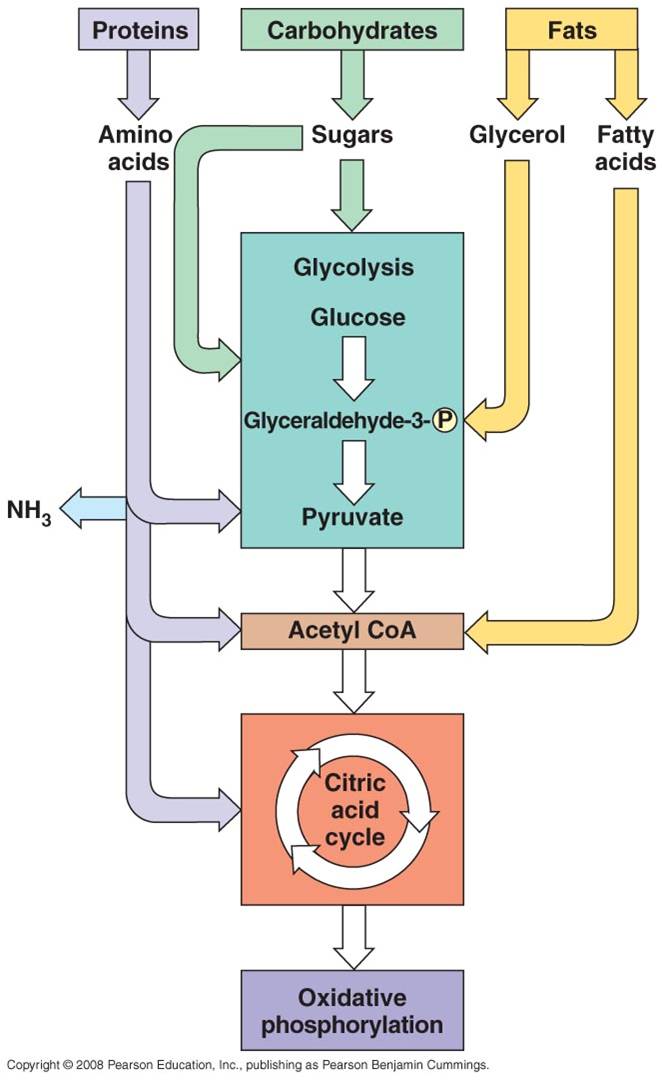
All four classes of biological molecules (carbo's, fats, proteins, and nucleic acids) are broken down for energy harvest. The process of carbohydrate metabolism, however, is the central process. Fats, proteins, and nucleic acids are broken into their monomers, these are modified, and then these products can be shunted into the carbohydrate digestion process. So, although we will focus on carbohydrate metabolism - and glucose metabolism in particular - you should appreciate that all other polymers can be broken down for energy harvest. And respiration not only harvests energy - respiration also provides the monomers needed by the cell to build its own biomolecules. So, when you digest protein, energy is harvested and the separated amino acids can be used by your cells to make your DNA-specified proteins. This is why a balanced diet is important - digestion of varied complex organic molecules provides the different monomers and other essential vitamins and minerals (often used as cofactors in reactions) that your cells require.
The metabolism of glucose can accur in the presence of absence of oxygen. The first step is glycolysis, in which the six-carbon sugar is split into 2 C3 molecules of pyruvate. The breaking of this bond releases a small amount of energy. In the absence of oxygen, fermentation occurs. The primary function of this "anaerobic" respiration is to recyclce some chemicals needed to keep glycolysis going. So, anaerobic respiration, including glycolysis and fermentation, breaks only a couple bonds and produces only a small amount of energy. In the presence of oxygen, the pyruvates can be completely oxidized. The C3 molecules are completely broken down into 3 one-carbon molecules of carbon dioxide. The complete breakdown of the the pyruvates releases much more energy. This is probably why aerobic organisms have come to dominate the planet - they harvest more energy from the food they consume, and can use this energy to survive and reproduce more effectively.
1. Glycolysis
a. the process:
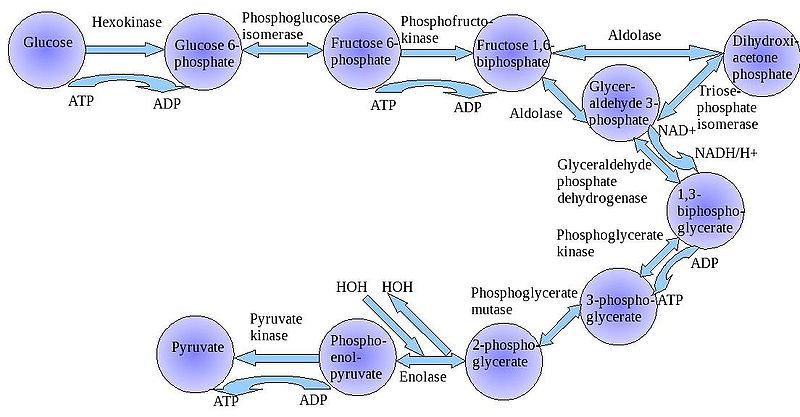 The
"splitting of glucose" (glyco-lysis) is probably an ancient metabolic
reaction; it is performed in the cytoplasm of ALL living cells from prokaryotes
to eukaryotes, and cells can perform this reaction in the presence OR absence
of oxygen gas. So, it seems likely that this was an important energy harvesting
reaction for ancient cells that lived before ~2 bya - before oxygen became abundant
in the oceans and atmosphere. As you can see in the flowchart, glycolysis is
not ONE reaction - it is a series of reactions catalyzed by a variety of enzymes.
For our purposes here, we will consider the primary "inputs" and "outputs"
of the entire reaction, rather than concerning ourselves with each step.
The
"splitting of glucose" (glyco-lysis) is probably an ancient metabolic
reaction; it is performed in the cytoplasm of ALL living cells from prokaryotes
to eukaryotes, and cells can perform this reaction in the presence OR absence
of oxygen gas. So, it seems likely that this was an important energy harvesting
reaction for ancient cells that lived before ~2 bya - before oxygen became abundant
in the oceans and atmosphere. As you can see in the flowchart, glycolysis is
not ONE reaction - it is a series of reactions catalyzed by a variety of enzymes.
For our purposes here, we will consider the primary "inputs" and "outputs"
of the entire reaction, rather than concerning ourselves with each step.
Through this series of reactions, the six-carbon glucose is modified and split into 2 C3 molecules of pyruvate. Glycolysis requires an input of energy to "get the reaction going". This activation energy is provided by 2 ATP. A phosphate is transferred from each ATP to the terminal carbons on the glucose. These phosphates destabilize the glucose, and also give it a charge - it will not diffuse back across the lipid bilayer. The splitting of the molecule releases energy and high energy electrons. Some of the energy is used to phosphorylate 4 ADP--> 4 ATP. Thus, although 2 ATP were used to start the reaction, there is a net gain of 2 ATP. The high energy electrons are accepted by an important molecule called NAD (nicotinamide adenine dinucleotide). With the acceptance of an electron, each NAD becomes negative charged (NAD-) and reacts with a H+ ion in solution - making NADH. So, NAD is a low energy form of the molecule, and NADH is a high energy form of the molecule.
So, for our purposes here, we can summarize glycolysis as:
glucose (C6) + 2 ATP + 2NAD ----> 2 pyruvate (C3) + 4 ATP + 2NADH
Oxygen is a very reactive gas - it oxidizes things - stripping electrons from other molecules and breaking bonds. Combustion is an oxidative process, and it can occur spontaneously, without an ignition source. So, if combustible material heats up above its ignition temperature, and if a strong oxidative agent like oxygen is present, it will ignite. Gasoline is a long hydrocarbon polymer. When raised above it's ignition point, it will combust. The gasoline will be oxidized to CO2 and H2O - and the breaking of the carbon-carbon bonds that occurs during this process will release energy. A gallon of gasoline contains ALOT of bonds and ALOT of energy; and if it is released all at once, the energy is difficult to control or use - you get an uncontrolled explosion. In a car's internal combustion engine, very small amounts of gasoline are ignited in sequence, by spark plugs, causing a little explosion in each cylinder that pushes the piston that turns the crankshaft that turns the wheels of the car. In a diesel engine, there are no spark plugs and no spark; the fuel ignites when the temperature exceeds its ignition point when placed under high pressure when the piston rises. By controling the reaction, by oxidizing just a little at a time, the energy released can be used to do work.
All organic molecules - sugars, nucleic acids, proteins, and fats - can be oxidized in the presence of a strong oxidative agent like oxygen gas. In fact, after oxygenic photosynthesis evolved and after iron precipitated out of suspension, when oxygen began to accumulate in the oceans and atmosphere, it was probably toxic to life. As described above, most of the most ancient forms of life - like archaeans - are obligate anaerobes and are still poisoned by oxygen to this day. Some life forms evolved specific enzymes and 'anti-oxidants' to protect themselves from the oxidative effects of oxygen gas, and they tolerated this new environment. Some of their descendants evolved a mechanism to use the oxidative effects of oxygen gas in a productive way - in a way that released the energy in organic molecules in controlled reactions where the energy could be harvested effectively and used to do chemical work. These aerobic organisms came to dominate the planet, probably in part because they could harvest more energy from the food they consumed; energy they could use to grow, survive, and reproduce. Aerobic respiration probably evolved about 2.0 billion years ago, in response to the increase in oxygen concentrations. Shortly thereafter, aerobically respiring bacteria were engulfed by other cells but not consumed; rather, the host cells used the ATP produced by these energetically efficient 'bacteria'. These host cells were the first eukaryotes, that evolved about 900 million years ago. Their energetic endosymbionts evolved into the mitochondria present in living eukaryotic cells. Like chloroplasts, mitochondria have their own bacteria-like chromosome and a bacteria-like double membrane system. In eukaryotes, the process of aerobic respiration occurs within these organelles. From these ancestral eukaryotes, some absorbed photosynthetic endosymbionts, too; these because the photosynthetic algae and their descendants, the plants. SO - PLEASE KNOW THIS: all eukaryotes (except a couple wierd protists like Giardia), HAVE MITOCHONDRIA - that includes protists, fungi, animals, and PLANTS. Photosynthetic eukaryotoes, the agae and plants, ALSO HAVE chloroplasts.
a. Overall Process:
- Pyruvates are broken down
into carbon dioxide.
- Energy that is released from
the complete breadown of the C-C bonds is used to make bonds in ATP (38).
- When bonds are broken, electrons
are released. They have to be accepted by another molecule. They are initally
accepted by NAD and FAD, which then take their "high energy" forms of NADH and
FADH2. Ultimately, they transfer this energy to ATP, give up
their electrons and H+, and are recylced as NAD and FAD. (That's important,
remember? We need to recycle that NAD so glycolysis - the first step in
this whole process - can continue.)
- Ultimately, the electrons
are passed to Oxygen O--, which then binds two hydrogen ions to balance charge
(forming water).
- Aerobic respiration is a
more complete breakdown of glucose, so it yields more ATP than glycolysis, alone
- In eukaryotes, this occurs
in a three step process in the mitochondria of cells.
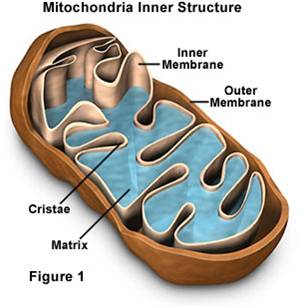 b.
Mitochondrial Structure:
b.
Mitochondrial Structure:
- Mitochondria have a double membrane
system like bacteria and chloroplasts, with an intermembrane space and matrix
within inner membrane.
- They have their own DNA, and they
replicate themselves by fission - they aren't 'made' by the cell.
- Given these observations, Lynn
Margulis hypothesized that these similarities were due to common ancestry, rather
than common environment. She raised this hypothesis as the endosymbiotic
hypothesis of eukaryote evolution, hypothesizing that eukaryotes acquired their
organelles by engulfing free-living bacteria and, rather than digesting them,
simply engulfed them and consumed their products (in this case the ATP that
the bacteria produce. The relationship is called symbiotic, because Margulis
hypothesized that the bacteria would also benefit by being in a stable environment
where the concentration of glucose was high (inside the cell).
- The most direct test of a hypothesis
of relatedness is DNA similarity. DNA only comes from parents, so similarities
imply a common source. When these tests were performed in the 1970's, her hypothesis
was confirmed. Additional tests with choloplasts and basal bodies (other
organielles in eukaryotes) also showed strong patterns of relatedness with free-living
bacteria. As such, we now refer to this tested model as the Endosymbiotic
Theory. We will describe this theory in more detail later in the term...
c. The Details:
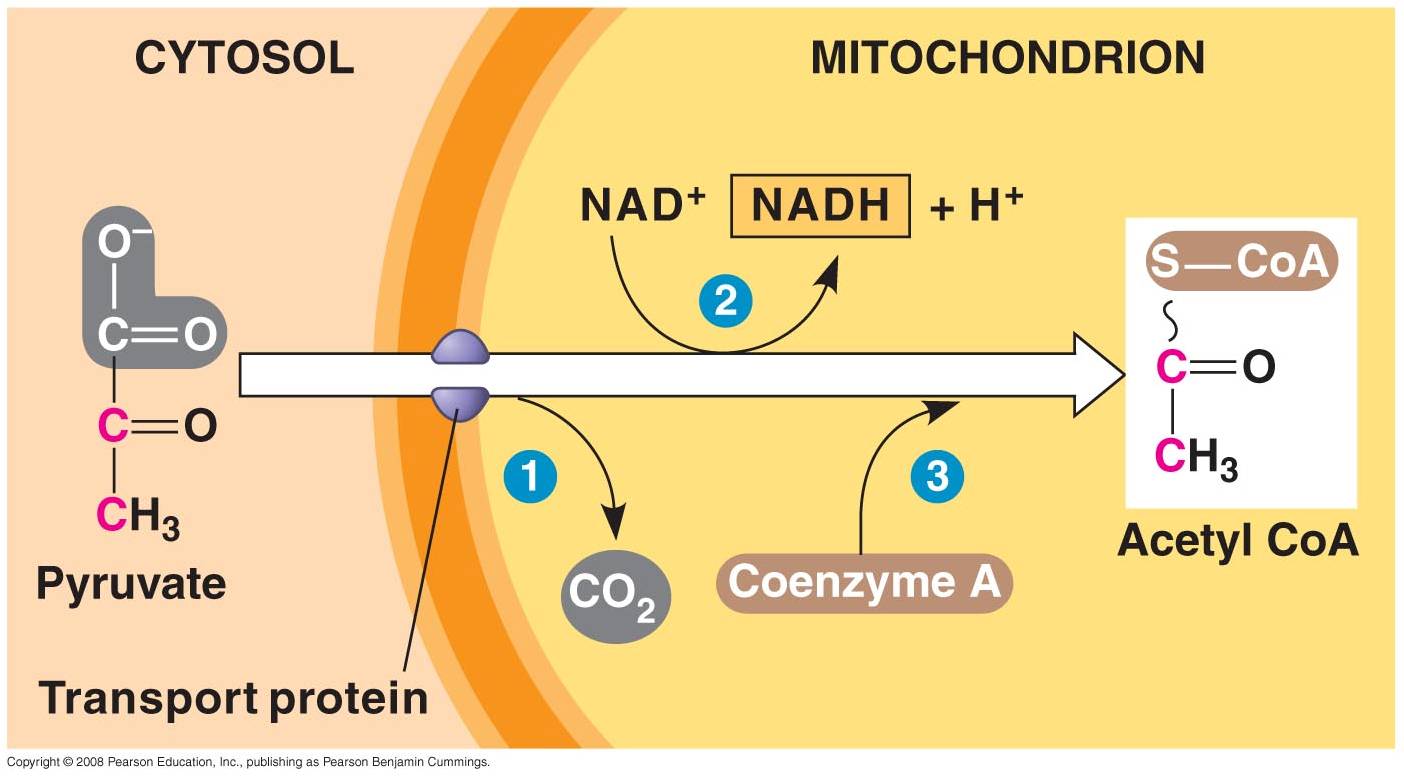 1. 'Gateway' Step:
1. 'Gateway' Step:
- Pyruvates cross both membranes
into the mitochondria and enter the 'matrix' - the cytoplasm of the organelle.
- Each pyruvate reacts with
a Coenzyme A molecule, and is split into a C2-CoA molecule and CO2.
(One C broken off).
- The electrons and energy
released are accepted by NAD, forming 1 NADH for each pyruvate used.
2. Krebs Cycle: 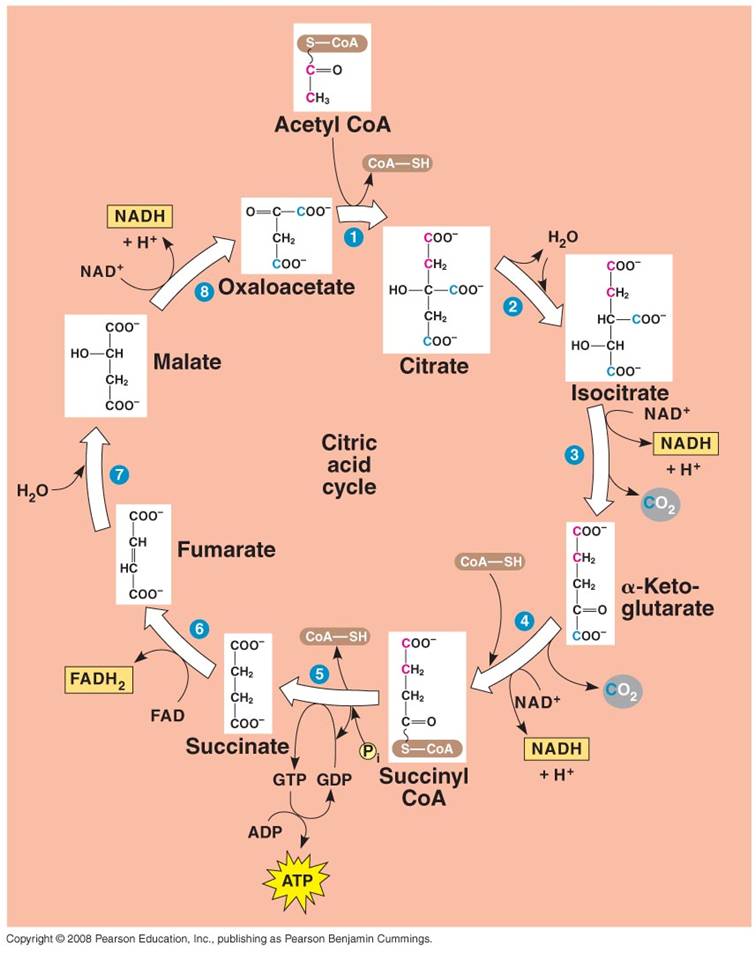
- Each C2-CoA reacts
with a C4 molecule (oxaloacetate).
- The C2 acetate
is transferred to the C4 molecule, forming a C6 molecule
of citrate. (CoA is released and recycled).
- Through a series of reactions,
the 2 'extra' C's are broken off as CO2 molecules and the C4
molecule is regenerated (Cycle).
- Some of the energy released
by the breaking of the C-bonds is used to make 1 ATP, 3 NADH, and 1 FADH2.
3. Electron Tranport Chain:
- Proteins nested in the inner
membrane of the mitochondria accept the electrons from NADH and FADH2
- The electrons are passed
from molecule to molecule, and some of the energy released is used to pump H+
ions across the inner membrane from the matrix to the intermembrane compartment
- Asteep concentration gradient
of H+ ions is formed... this represents chemical potential energy.
- When the ions flow through
protein channels associated with ATP-synthesizing enzymes in the membrane, this
potential energy is transformed into chemical energy in bonds between ADP and
P, making ATP.
- When the electrons reach
a low energy state, they are accepted by oxygen in the matrix and H+ ions react
with the O-- to form water. This is the ONLY use of oxygen in the process
- as an electron acceptor.
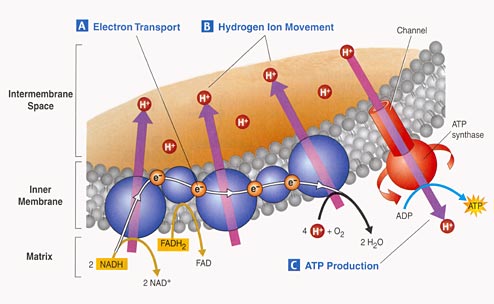
- 34 to 36 ATP are made from the energy tranferred from NADH and FADH2 molecules produced in the Krebs Cycle.
a.
Fats:
- Glycerol broken from fatty acids; glycerol (3C) fed into glycolysis and
are modified into pyruvates (C3). - the Fatty Acids are broken
down into C2 groups that are modified to react with CoA - they
are shuinted to the Krebs Cycle - these reacts are reversible, so if there
is a surplus of C2-CoA, it can react to form fatty acids ---> energy
consumed in carbo's can be stored as bonds in fat.
b.
Proteins:
- Broken into Amino Acids which, depending on their structure, can be shunted
into glycolysis, modified into pyruvate, or broken into acetate (C2).
- In all cases, the amine groups are cleaved, producing ammonia (NH3)
as a toxic waste. In mammals, this is converted into urea which must be diluted
in water for removal from the body (urine). Reptiles and birds convert it
to uric acid, which is expelled as a paste that does not require as much water
for dilution.
c.
Nucleic Acids: - ribose can be metabolized after coversion to
glucose.
Study Questions:
1) What is the structure of a fatty acid? A triglyceride?
2) What is the monomeric unit of proteins? Draw one, without specifying the variable group.
3) Show how two amino acids are linked together. What is the name of the reaction?
4) Diagram the parts of an RNA nucleotide.
5) Show how two nucleotides are linked together by dehydration synthesis reactions.
6) Why does the purine - pyrimidine structure relate to the complementary nature of double-stranded DNA?
7) Draw a DNA double helix, showing three base pairs and the antiparallel nature of the helices.
8) Describe the higher levels of eukaryotic chromosome structure, including the terms nucleosome and solenoid.
9) List three differences between prokaryotic and eukaryotic cells.
10) Why is the lipid bilayer a barrier to water soluble molecules?
11) Describe diffusion, facilitated diffusion, and active transport.
12) Describe glycolysis.
13) What happens in the gateway step?
14) What happens in the krebs cycle?
15) What happens in the elEctron transport chain? Explain chemiosmosis.
16) How is oxygen involved in the process of aerobic respiration?