The standard working model of earth formation goes like this. Our solar system probably formed as a gas nebula began to condense and spin, perhaps because the explosion of a nearby supernova caused the gas cloud to collapse on itself or it came under the gravitatinal field of a passing star. As this gas cloud collapsed and began to rotate, the center continued to collapse, form a strong gravitational field, heat up, and ultimately radiate light and energy (sun). Under this gravitational field, the rest of the spinning gas cloud flattened into a protoplanetary disk. The debris orbiting the sun at the same distance collided and formed larger bodies called 'planetissimals', which continued to collide and accrete. As these objects became larger, their masses and gravitational fields increased and they swept more material up as they orbited the sun - eventually resulting in a few large bodies at different distances (planets). Temperatures were too hot near the sun for volatile molecules (like water) to condense, so only matter with higher melting points - like metals, condensed to form the inner, rocky planets. Volatile molecules could condense farther from the sun, creating the huge gas giants of the outer solar system. The gravitational fields of Jupiter and Saturn were so large that they disturbed the formation of any large bodies nearby - so the planetissimals in the asteroid belt between Mars and Jupiter failed to form planets or were ripped apart by gravitational forces of Saturn and Jupiter. Our solar system includes the Sun, the planets (Mercury, Venus, Earth,Mars, Jupiter, Saturn, Uranus, and Neptune), moons that orbit the planets, and 'dwarf planets' that orbit the sun but are smaller than our moon (like Pluto and Makemake--the largest object in the asteroid belt). The Sun and the planets are so massive that they exert large graviational fields that attract one another, keeping the planets in orbit around the most massive object (Sun), even at extraordinary distances that are really incomprehensible. Consider the model discussed in the powerpoint to appreciate the force of gravity generated by these huge objects: if the Sun were the size of a basketball, Pluto ( 0.02 inch in size in this model) would be held in an orbit that is, on average, 4/5's of a mile away.

Mars is the fourth planet from the sun (Earth is third) and second smallest planet in the solar system; only Mercury is smaller. It is likely that the growth of Jupiter prohibited planetissimals from accreting onto Mars or condensing into a larger separate planet—they continue to orbit as the unconsolidated ‘asteroid belt’ between the orbits of Mars and Jupiter. Mars is about ½ the size of earth in radius and equatorial circumference. Its “day” is about 24 hrs, but it takes 1.88 earth years to orbit the sun. Although Earth temperatures range between -88 and 58oC (136oF), Mars ranges from -153 to 20oC (68oF). All surface water is bound up in a polar ice cap, although erosional landscapes suggest that liquid water did flow over the surface in the past. Venus is the second planet from the sun and is only slightly smaller (95%) than the Earth. It spins slowly (and opposite to Earth), taking 117 earth days to complete one rotation, and only spins a bit more than once per year (225 Earth days). Surface temperatures reach 462oC (~900oF). Venus is constantly clothed in clouds because most compounds vaporize at these temps; the clouds force a “runaway greenhouse effect” that traps heat. All of the scant water exists as water vapor. These temperature differences contribute to the first major difference between Earth and these planets is that Earth is covered (71%) by liquid water.
All three planets (and Mercury) are “rocky”, as opposed to the gas giants in the outer solar system. Mars and Venus have very similar atmospheres, composed largely of CO2 with a small amount of N2. In contrast, Earth has little CO2. The reduction in this quantity makes the proportional representation of N2 increase dramatically. And of course, there is O2 in the Earth’s atmosphere, which is all but absent in the atmospheres of the other planets.
Earth |
Venus |
Mars |
|
CO2 |
0.035% |
96% |
95% |
N2 |
77% |
3.5% |
2.7% |
H2O |
1% |
0.01% |
0.007% |
Ar |
0.93% |
0.007% |
1.6% |
O2 |
21% |
trace |
trace |
1. conventional matter - defined as a substance that has mass and occupies space - consists of atoms.
2. there are 94 different types of naturally occurring atoms - defined by differences in the number of protons in the nucleus. These are the naturally occurring elements which have different properties (mass and charge, for instance) as a consequence of their different number of protons. Atoms are also defined as the smallest unit of a pure substance (one element) that retains the properties of that substance and cannot be subdivided further by chemical means.
3. compounds are substances composed of two or more types of elements in a fixed ratio, with a particular structure/spatial arrangement maintained by chemical bonds (ionic or covalent). So, NaCl (table salt) is an ionic compound. H2O (water) is a covalent compound.
4. molecules are substances of two or more atoms bound together by covalent bonds. So, it is inappropriate to refer to a 'molecule' of NaCl (which is ionic). Two atoms of hydrogen bound together by a covalent bond is a molecule of hydrogen. It is not a compound, as it only contains one type of element. For compounds consisting of covalently bound atoms, a molecule is the smallest unit of the compound that maintains the properties of that compound. So, we can have one molecule of water, which is also a compound.
We will use a very simplistic 'Bohr' model of atomic structure, emphasizing the particulate nature of matter (rather than the wave nature) and excluding a consideration of quarks. For a more detailed account, feel free to see this link from the University of Oregon and Wikipedia.
1. atoms have a nucleus containing protons (mass ~ 1 atomic mass unit; elementary charge = +1 ) and neutrons (mass ~ 1 atomic mass unit; elementary charge = 0). The number of protons defines the type of atom - the element - and it's atomic number. The mass of an atom is largely determined by the mass of protons and neutrons. Although all atoms of an element have the same number of protons, the number of neutrons can vary. These atoms with variable numbers of neutrons are called isotopes. Those with an excess number of neutrons are less stable and will loose them over time. These are radioisotopes, and they emit energy when they loose a neutron. They loose them at a constant rate, so you can date how old they are by how many have changed to the more stable state. Atoms with unequal numbers of protons and electrons have a net charge and are called ions.
2. the nucleus is surrounded by a cloud of electrons (mass ~ 0; elementary charge = -1), represented as shells and orbitals: Shells 1, 2, 3 have 1, 4, 9 orbitals, 'containing' a maximum of 2, 8, 18 electrons, respectively. The orbitals are 1000's of times the width of the nucleus, so an atom (and hence, all matter) is mostly space. For comparison, if you envision the nucleus of a carbon atom as a basketball (about 12 inches in diameter), the outermost electrons would be orbiting 5 miles away.
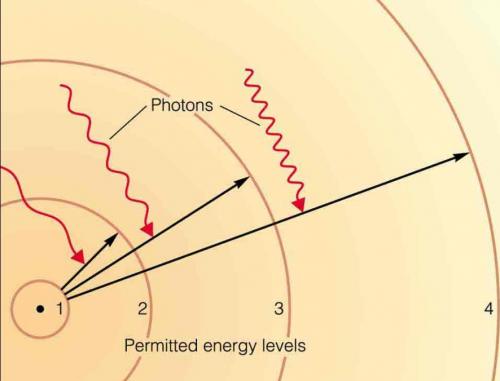 3. the distance between the orbital and the nucleus correlates with the energy of the electron (in terms of a wave function).
3. the distance between the orbital and the nucleus correlates with the energy of the electron (in terms of a wave function).
4. as electrons gain energy, they 'orbit' farther from nucleus. Without an input of energy, an atom will lose energy and approach it's lowest energy state, occupying the closest 'unoccupied' orbital available.
5. changing orbitals - and energy states - is a discrete process. Electrons must gain or lose discrete amounts of energy (quanta) to change position to another orbital.
6. Binding properties are largely governed by the number of 'valence' electrons in the outermost orbital/shell. They achieve greater stability when the outermost orbitals/shells are full. They achieve this stability by losing, gaining, or sharing electrons with other atoms. For the first three shells, the valence electrons are 2, 8, and 8... hence the 'octet rule'.
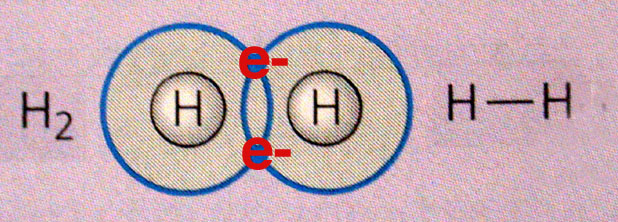 1. Covalent Bonds
1. Covalent Bonds
Atoms share electons in pairs, each contributing one electron to the shared pair: H2, H2O, etc. These are the primary bonds in biologically important molecules - they can be non-polar (shared evenly) like H2 - or polar (shared unevenly...creating a charge difference across the molecule - as in water, where the shared electrons are held more tightly by the larger oxygen nucleus, pulling the electron cloud off the hydrogen nucleus, revealing some of its positive charge).
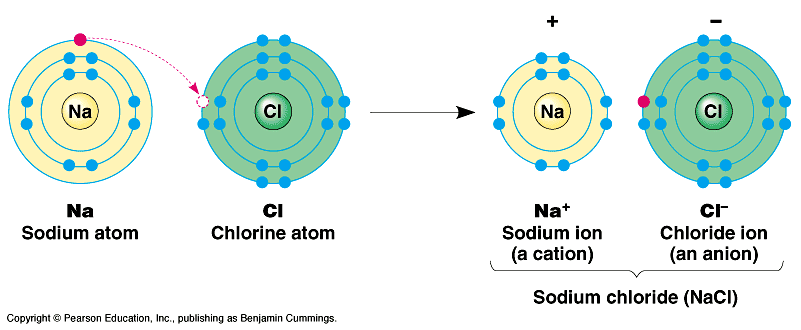
2. Ions and Ionic Bonds
Electrons can be transferred completely from one atom to another. This creates charged particles (ions) which may then be attracted to one another based on their opposite charge. (NaCl)
3. Hydrogen Bonds
These are weak ionic bonds, weak forces of attraction between partially charged regions of different molecules. These partial charges arise because, in polar covalent bonds, the unequal sharing of electrons gives one atom a slight negative charge and the other a slight positive charge. This is common when hydrogen is invovled because, as the smallest atom, all others elements will attract the shared electrons more forecefully.
1. Water’s molecular structure
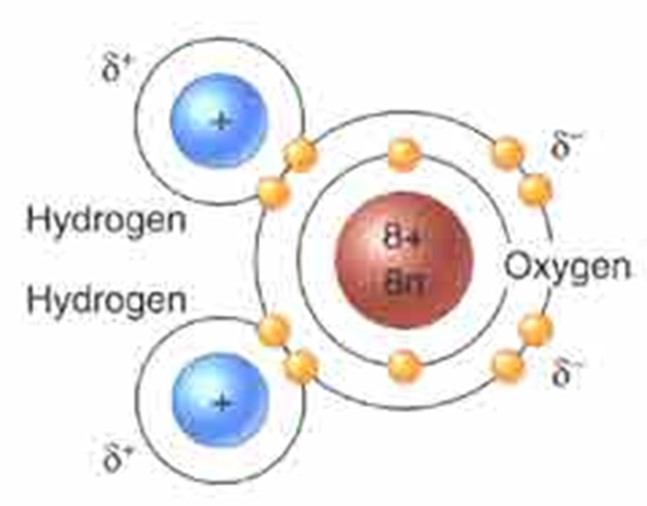 Water has numerous properties that affect Earth (and life). Water is composed of one oxygen atom, with 8 protons, and 8 neutrons in the nucleus, orbited by 8 electrons. There are 2 electrons orbiting in the first “shell” and 6 in the second. The second shell can hold 8, and atoms are most stable if the outer shell is full ('octet rule'). So, oxygen will interact with other atoms and share pairs of electrons to “fill” its outer shell. This sharing of electrons between atoms is called a “covalent bond”. One oxygen atom will form single covalent bonds with two hydrogen atoms, each that has only one electron in its first shell. By sharing pairs of electrons, each atom fills its outer shell and is stable.
Water has numerous properties that affect Earth (and life). Water is composed of one oxygen atom, with 8 protons, and 8 neutrons in the nucleus, orbited by 8 electrons. There are 2 electrons orbiting in the first “shell” and 6 in the second. The second shell can hold 8, and atoms are most stable if the outer shell is full ('octet rule'). So, oxygen will interact with other atoms and share pairs of electrons to “fill” its outer shell. This sharing of electrons between atoms is called a “covalent bond”. One oxygen atom will form single covalent bonds with two hydrogen atoms, each that has only one electron in its first shell. By sharing pairs of electrons, each atom fills its outer shell and is stable.
However, since the nucleus of the oxygen atom (with 8 positively charged protons) attracts the negative electrons more than the one proton in each hydrogen atom, the electron pairs are not shared evenly; they are held more closely to the oxygen, so it is slightly negative… the hydrogens have their negativity pulled away from their proton, revealing a part of its positive charge. So, there is a ‘polarity’ to a water molecule – a positively charged end and a negatively charged end. This is a critical property: WATER IS POLAR. Because water is polar, it forms hydrogen bonds with itself. If the free energy in the system is high, then the molecules will be too exited to bond with one another and the sate will be water vapor. If the free energy is low, then the molecules will all bind, forming a lattice of molecules in solid ice. In between, (0 - 100oC) bonds break and reform and the molecules shift connections but are rarely completely free--existing as liquid water.
2. Water is called the “universal solvent” - Charged compounds (polar or ionic) dissolve in water
Many materials have charges, sometimes for the same reason that water does. Sugar molecules are partially charged, like water, and so sugar molecules can become separated from themselves in water, binding to water instead of one another.
Some materials (atom or molecule) have an unequal number of protons and electrons, and thus have a “complete” charge of -1 or +1, depending on whether the surplus is an electron or proton. They can have larger charges (like +3), too. Since they are charged, they can also attract and interact with water molecules and thus become separated (dissolve).
Table salt (sodium chloride) is a typical example of an “ionic compound”, composed of Na+ and Cl- atoms that are attracted to one another because of their opposite charges. Typically, these oppositely charged ions attract one another in a lattice/crystal. But placed in water, the ions become separated and attracted to water…. Dissolving.
Think of the electrons shared between the large oxygen nucleus and each small hydrogen nucleus (1 proton) as a “bed sheet” of electronegativity. When the larger oxygen nucleus rolls itself up in the sheet, it pulls the sheet partial off the hydrogen nucleus, revealing a portion of the proton’s positive charge. Sometimes, the oxygen pulls the sheet completely away from the hydrogen; now they aren’t sharing the electron-pair at all; the hydrogen nucleus is ‘naked’ – just 1 proton – existing as an independent H+ ion. The oxygen (and the other hydrogen) now have an extra electron and are a negatively charged hydroxide ion. The water molecule has “dissociated” into H+ and OH- ions. Now, of course, these oppositely charged ions are attracted to one another and they will re-associate. Water molecules are constantly splitting and rejoining in solution. In pure water, 1 in 10,000,000 (10 million) water molecules is dissociated at any one time. This equals a concentration of 1 x 10-7, and the negative of the exponent that describes the concentration of free H+ ions is an important characteristic of a liquid… it is called the pH (sort of the “percent Hydrogen”). In this case, pure water has a pH = 7.0. Hydrogen ions are very reactive. So, fluids with high concentrations of free H ions (low pH) are very reactive. Consider what happens between Cl (with 17 P and 17 e) and Hydrogen (1 p, 1 e). They share a pair of electrons and fill their outermost shells, making HCl (Hydrochloric acid). But, since the Cl is SO MUCH BIGGER (twice the pull of oxygen’s 8 protons), it rips the electron pair away from Hydrogen more frequently. In fact, the concentration of dissociated HCl molecules in solution, and thus the concentration of free H+ ions, is not 1 in 10 million like with water, it is 1 in 100! This is represented in exponential notation as 1 x 10-2, and we say that HCl has a pH = 2.0.
4. Water weathers rock, putting ions into solution
This is important because ROCKS can dissolve in water. Water also erodes rock, breaking pieces off mechanically and putting them in suspension and carrying them to other places. These physical and chemical processes of rock breakdown are called “weathering”. Water gets rocks moving and puts ions in solution where they can react with other chemicals.
One way that water dissolves rock is by “cation displacement”.
The most abundant minerals in the Earth’s crust are feldspars – they make up 60% of the Earth’s crust. Chemically, they are KAlSi3O8, NaAlSi3O8, and CaAlSi3O8. K+, Na+, and Ca+2 are positively changed ions. In the presence of water, the dissociated H+ ions ‘displace’ these other cations (positive ions), which are soluble in water because of their charge, leaving the silicate behind.
CO2 is soluble in water, and it combines with water to form carbonic acid. Acids dissociate, giving up their H+ ions, as we know. Carbonic acids in the soil are much more important in dissolving rock than water, as they have a pH of 5.6 (more acidic – a higher tendancy to give up H+). Carbonic acid dissociates, forming bicarbonate and carbonate. The carbonate reacts with Ca+2 in solution, forming “calcium carbonate” which settles out of the water as limestone, or precipitates when the water evaporates. This is the “abiogenic” (without life) process that forms limestone. Most limestone forms biogenically, though, as we will see.
B. The Earth’s crust is recycled by tectonic activity
Mars is too small to generate the internal heat necessary for plate tectonics. Plate tectonics recirculates the Earth’s crust, taking some carbon from the atmosphere, that has been deposited in sediments, deep into the mantle. Venus is large enough to generate tectonic activity, but the crust is so hot that it “melts” and heals rather than forming plates that can be forced under one another, as on Earth. So it melts and crusts up, but doesn’t form plates. So, as a consequence of liquid water on Earth, CO2 in the atmosphere is dissolved, where it reacts with water and calcium and precipitates as limestone, which can be transported by plate tectonics deep into the Earth for long-term storage. Volcanoes return carbon to the atmosphere as carbon dioxide, but there is a net transfer to the lithosphere.
C. The Effects of Life
 1. Biogenic Limestone Formation
1. Biogenic Limestone Formation
Many marine organisms, including some types of photosynthetic algae (Coccolithophores), Molluscs (clams, oysters, mussels, snails), and corals absorb calcium and carbonate and form calcium carbonate to make their shells. When they die, these shells fall to the bottom of the ocean, accumulate, and under pressure are turned to limestone. Under enough pressure and heat, they metamorphose into marble. 10% of the Earth’s rock is limestone, and most of this was produced organically (biologically). As CO2 is converted to limestone, more CO2 dissolves in water. So, life sucks CO2 out of the air and into the oceans, converting it to limestone.
2. Photosynthesis
 As we will learn in more detail later, photosynthesis typically consists of two major reactions: the “light dependent” and “light independent” reactions. In the light dependent reactions, organisms transform radiant energy of sunlight, carried by photons, into chemical energy in chemical bonds, carried by electrons. So, in sunlight, photosynthetic organisms trap convert light energy into chemical bonds…typically by binding a phosphate (PO4) to adenosine diphosphate (ADP) to make ATP (adenosine triphosphate). To get the electrons that carry this radiant energy, the organisms split water molecules… the freed oxygen atoms from 2 water molecules unite, to form a molecule of oxygen gas (O2). So, photosynthetic organisms split water to harvest electrons, and oxygen gas is released as a waste product. All of the oxygen gas in our atmosphere (21%!!!) was produced by this process.
As we will learn in more detail later, photosynthesis typically consists of two major reactions: the “light dependent” and “light independent” reactions. In the light dependent reactions, organisms transform radiant energy of sunlight, carried by photons, into chemical energy in chemical bonds, carried by electrons. So, in sunlight, photosynthetic organisms trap convert light energy into chemical bonds…typically by binding a phosphate (PO4) to adenosine diphosphate (ADP) to make ATP (adenosine triphosphate). To get the electrons that carry this radiant energy, the organisms split water molecules… the freed oxygen atoms from 2 water molecules unite, to form a molecule of oxygen gas (O2). So, photosynthetic organisms split water to harvest electrons, and oxygen gas is released as a waste product. All of the oxygen gas in our atmosphere (21%!!!) was produced by this process.
In the light independent reactions, the chemical energy now present in the new bonds in ATP is converted into a more stable bond between carbon atoms. So, ATP is “broken” and the energy released is used to make bonds between carbon atoms. Where does the carbon come from? CO2 in the air.
So, photosynthetic organisms use the ATP made in the light dependent reactions to link 6 CO2 molecules together, making a glucose molecule that has six carbons. Photosynthesis contributes to the two major differences between the Earth’s atmosphere and those of Mars and Venus: it produces the O2 and sucks CO2 out of the atmosphere and makes glucose.
IV. The Biosphere
The biosphere is a critical component of the Earth system--when it changes, the way the Earth works changes, too, reflected by obvious changes in atmospheric composition. If we are concerned about these changes to the atmosphere and the global warming, sea level rise, increased fires and storms that follow, we might want to protect and sustain the biosphere so it can do its job. Well, if we want to sustain it, and maintain its function, we have to know how it works and why it works the way it does. What philosophical approach seems relevant here? How might we learn why it works the way it does? Reductionism dictates that we should describe what it is made of--that might help us understand how it works, and help us maintain these functions.
A. What is the BIOSPHERE?
 The biosphere is ALL LIVING THINGS: you, me, the trees outside, the squrrels, the mushrooms, the fish and the algae. It is the 2 million different species of living things that compose the known living world. The periodic table has only 92 naturally occurring elements; the biosphere has over 2 million species, interacting in complex ecosystems. We have just begun to understand how this DIVERSITY affects how the biosphere works. But you cannot appreciate the biosphere without appreciating these parts--what they are and how they work. This is the domain of BIOLOGY... Not environmental science and not sustainability science. If you want to sustain .the biosphere, then you must understand and study the biosphere. That is what BIOLOGY does, at levels from cells to the BIOSPHERE.
The biosphere is ALL LIVING THINGS: you, me, the trees outside, the squrrels, the mushrooms, the fish and the algae. It is the 2 million different species of living things that compose the known living world. The periodic table has only 92 naturally occurring elements; the biosphere has over 2 million species, interacting in complex ecosystems. We have just begun to understand how this DIVERSITY affects how the biosphere works. But you cannot appreciate the biosphere without appreciating these parts--what they are and how they work. This is the domain of BIOLOGY... Not environmental science and not sustainability science. If you want to sustain .the biosphere, then you must understand and study the biosphere. That is what BIOLOGY does, at levels from cells to the BIOSPHERE.
Of the 2 million species we have given a name to (like Homos sapiens or Drosophila m.elanogaster), about HALF--fully 1 million--are insects. Another 25% are other invertebrate species: other arthropods like crustaceans, and other phyla like the molluscs, segmented worms (Annelida), flat worms (Platyhelminthes), round worms (Nematoda), Cnidarians (corals and jellyfish), sponges, Echinoderms (starfish), and many more. Only 5% of the species are vertebrates: fish, amphibians, reptiles, birds, and mammals--the things most people think of as 'animals'! 20% of the named species are plants, and there are small fractions of fungi, protists, and bacteria. Aside from the insects (which are terrestrial), most animal species are marine organisms. We have probably found most the big stuff that lives with us on land; most of what is unnamed is marine and small. A quick walk through the insects shows us that they are diverse because there are differnt species that live in very differnt ways--they have different ecologies and contribute to their ecosystems in differnt ways. Unfortunately, alhtough we have named 1 million species, we know NOTHING about most of them. We collected them, and have yet to study how they fit into this extraordinary "machine" of the biosphere.
B. What does Biodiversity DO?
But, as a result of describing this biodiversity, we have begun to understand what biodiversity DOES (reductionism).
1. Diversity increases productivity
'Productivity' is the amount of biomass produced... typically, we measure 'primary productivity', which is the amount of plant biomass produced. Usually, this is standardized per unit area (m2) and per unit time (year). This 'primary productivity' is the energy harvest from sunlight and trapped in chemcial bonds in plant molecules: glucose, cellulose, and all the plant proteins, carbohydrates, lipids, etc. In other words, 'primary productivity' is the food upon which all other life forms--including our own--depends. More diverse systems can be more productive than less diverse systems--even less diverse systems containing only the most productive single species. This occurs because of niche compatability and positive effects. Let's consider an example:
Consider a set of agricultural plants that vary in productivity, like corn, squash, and beans. Per unit area, under ideal growing conditions and densities, corn is the most productive species. So, you might conclude that, to produce the most food, you should plant your entire field just in corn. How can adding less productive species increase total food production over a monoculture of the most productive species? This is how.
- Niche complementarity: If you plant less corn, you may be able to plant squash at the base of each corn plant. The squash and corn use different nutrients, and won't compete as strongly as two corn plants placed this lose together. So, although you get less corn, the amount of squash produced can over-compensate for this lower corn production, because they don't have the same ecological requirements... their 'niches' are complementary ('fitting together', not 'overlapping').
- Positive effects: Now, suppose you add beans to the mix. Beans are legumes--and they have bacteria in root nodules that 'fix' nitrogen. Although our atmosphere is 78% nitrogen gas, N2, the atoms are held together by the strongest bond in nature: the triple bond. No animals or plants have enzymes that can break this bond, and thus make allow the nitrogen to bond to other atoms like hydrogen, carbon, or oxygen... as it needs to to make DNA and amino acids (in proteins). So, all animals and plants must get their nitrogen from their food or the decmposed nutrients in the soil. There are a few bacteria, however, that can break N2, and link N to H and O ('fixing' it to H or O), making it available to plants as a soil nutrient. Thus, these nitrogen-fixing bacteria 'fertilize' soils. They infect legumes and grow, feeding the legume but also raising the level of available nitrogen in the soil. SO! By planting beans, the corn grows a little better and you get more corn. The squash grows a little better and youget more squash. And, you get the beans!
So, by adding 'less productive' crops to the system, you actually get MORE food than the monculture of the most productive plant. When we look at the productivity of entire ecosystems, the productivity correlates with diversity: the most diverse terrestrial systems (rainforests) and marine systems (coral reefs and algal beds) are also the most productive. So, diversity is FUNCTIONALLY IMPORTANT to how the biosphere works.
2. Diverse ecosystems provide economically valuable services
The biosphere cycles energy and matter through the Earth system, and thus cleans our air and cleans our water, in addition to providing food (above). In addition, ecosystems protect our manmade systems from variation in nature: they act as storm breaks, they absorb excess water in coastal storm surges, they reduce erosion, they moderate the climate. In a 1997 article, Costanza and coauthors quantified th economic value of these services. It exceeded the GNP of all countries, together. In other words, if we had to replace these services...like constructing desalinization plants to make fresh water... it would take more money than all our economies generate. The biosphere is doing these things for free.
3. Aesthetic value
Finally, the biosphere provides the diverse contexts in which our diverse cultures have evolved. We draw sybols from nature to communicate ideas. Indeed, even how we think is a function of our environment. A rich and diverse nature enriches our culture and our lives.
C. Why Preserve Biodiversity?
 So, diversity does some really important things. But do we really need to protect all of it? There are 350,000 species of beetles; does each species do something uniquely important? Probably not; there is alot of redundancy in nature, and nature can respond to changes like the loss of a species. Over the last 200 years, we have hundreds of examples of species that went extinct, yet the biosphere has not collapsed. The problem is this: although we have given names to 2 million species, we know nothing about most of them. Ecology is a young science, and we don't really know how the parts of an ecosystem--the species--all fit together to cause the system to work (reductionism). So, we don't know which species are more important than others. We are 'tinkering around' with these systems, killing off this species and that one indiscriminantly, without knowing what we are doing. As Aldo Leopold, a famous ecologist of the 20th century said, "the first rule of the tinkerer is to save all the pieces." If you are tinkering around with a toaster and you want to have any hope of putting it back together again so it works, you better keep all the pieces.
So, diversity does some really important things. But do we really need to protect all of it? There are 350,000 species of beetles; does each species do something uniquely important? Probably not; there is alot of redundancy in nature, and nature can respond to changes like the loss of a species. Over the last 200 years, we have hundreds of examples of species that went extinct, yet the biosphere has not collapsed. The problem is this: although we have given names to 2 million species, we know nothing about most of them. Ecology is a young science, and we don't really know how the parts of an ecosystem--the species--all fit together to cause the system to work (reductionism). So, we don't know which species are more important than others. We are 'tinkering around' with these systems, killing off this species and that one indiscriminantly, without knowing what we are doing. As Aldo Leopold, a famous ecologist of the 20th century said, "the first rule of the tinkerer is to save all the pieces." If you are tinkering around with a toaster and you want to have any hope of putting it back together again so it works, you better keep all the pieces.
D. How is this Diversity Produced?
To preserve diversity, we must understand how it is produced and maintained. Charles Darwin was the first to conclude that diversity is produced through the process of radiatinal evolution: species 'split' over time, giving rise to multiple new species. As he said at the close of Origin of Species, "There is grandeur in this view of life, with its several powers, having been originally breathed a few forms or into one; and that, whilst this planet has gone cycling on according to the fixed law of gravity, from so simple a beginning endless forms most beautiful and most wonderful have been, and are being, evolved." This course will now examine how this happens.
Study Questions:
1. How did the solar system form? What is the order of the planets, from the sun outward?
2. What are the compositions of the atmospheres on Earth, Venus, and Mars with respect to CO2, O2, and N2?
3. Draw the structure of a water molecule, showing all electrons for the atoms. Denote shared electron pairs.
4. What is a polar covalent bond and why does it form?
5. Why does water’s polarity make it a good solvent?
6. What does it mean to say that water “dissociates”, and what does it mean to say that pure water has a pH = 7.0?
7. What effect does water have on rock, and how does it exert this effect?
8. What effect does water have on CO2 in the atmosphere? Explain the formation of abiogenic limestone.
9. Describe biogenic limestone formation.
10. Outline the process of photosynthesis, and state the two ways it affects the composition of the atmosphere.
11. How and why do CO2 and O2 levels vary over the course of a year?
12. How and why is CO2 increasing and O2 decreasing from decade to decade? Describe two reasons.
13. How many species have been identified by science, and what fractions are comprised by insects, other invertebrates, plants, and vertebrates?
14. Describe the two ways that biodiversity can increase productivity.
15. What are ecosystem services?
16. What is a 'keystone species' (look it up somewhere)? Describe how sea otters act as keystone species.