Cell Biology
I. Overview
II. What Cells are Made of: Biologically Important Molecules
III. Membranes: How Matter Get in and Out of Cells
A. Membrane Structure
All living cells are bounded by
a membrane composed of a phosopholipid bilayer. Proteins are present on and
within each layer, and some also cross all the way through the membrane.
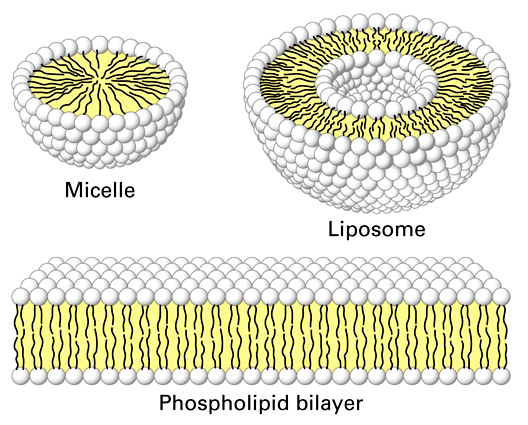 1.
Phospholipid Bilayer:
1.
Phospholipid Bilayer:
- phospholipids have hydrophobic
fatty acid tails and hydrophilic phosphate group "heads" (charged).
In an aqueous solution, phospholipids will form micelles (single layer spheres)
and bilayers (two layer spheres and films) as a function of the hydrophobic
and hydrophilic nature of these molecules. These spatial orientations surround
the hydrophobic fatty acid 'tails' with the hydrophilic phosphate 'heads' that
interact with the polar water molecules in the solution. When arranged as a
bilayer, they separate the internal aqueous solution of the cell from the external
aqueous environment. Because the principle boundary is the hydrophobic, non-polar
layers of fatty acids, the bilayer is permeable to lipid-soluble materials but
not water soluble (polar and ionic) materials unless they are very small. These
bilayers are also very dynamic or "fluid"... the phospholipids are
moving laterally all the time, like the lipids in a soap bubble.
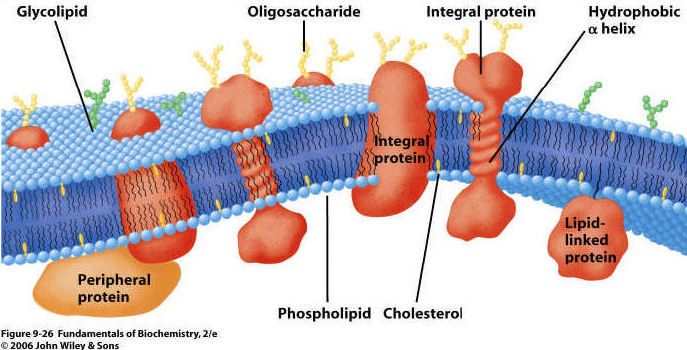
2. Proteins arepresent on both
inner and outer surfaces, and also extending through the lipid bilayer.
3. Carbohydrates on the surface;
usually attached to proteins and forming a "glycoprotein"
B. Membrane Function
1. Semi-permeable barrier:
If a substance dissolves in water, then it is polar or ionic (amino acids, sugars,
nucleic acids). So, it does not cross the non-polar lipid bilayer .
Lipid-soluble materials cross membrane rapidly (fats, steroids, CO2, O2).
So, a lipid layer is a great barrier for separating two aqueous solutions and
the dissolved solutes they contain (those inside and outside the cell).
2. Transport: matter does cross
the membrane. However, unless it is a small or non-polar molecule, it cannot
cross the lipid bilayer "on its own". Rather, crossing the membrane
must be assisted by a protein. These proteins may be rather unselective "tubes",
or they may be specific for the transport of a particular class of molecules.
By using proteins as 'gates' the cell gains control over the composition of
its cytoplasm. There are three ways that material can cross a membrane:
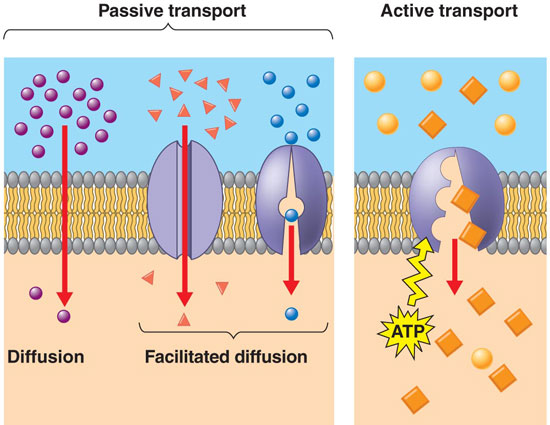
a.
Diffusion: As a consequence of the random movement of molecules,
molecules will disperse from areas of high concentration and move to areas
of lower concentration. So, when you uncork a bottle of perfume, the molecules
diffuse through the room from high concnetration at the bottle to low concentration
in the rest of the room. Molecules may cross membranes as they move in this
manner, moving from areas of high concentration on one side of the membrane
to areas of lower concentration on the other. With respect to the lipid bilayer
in living membranes, non-polar molecules and some small molecules can diffuse
directly through the lipid bilayer. The movement of CO2 and O2
across the membrane happens by diffusion. Every molecule moves by diffusion
in response to its own concentration gradient.
The movement of water across
a membrane, in response to its water potential, is called osmosis (video).
Although water is polar, it is also a small molecule. So, it can cross the
lipid bilayer directly, or through specialized protein channels called 'aquaporins'.
Water potential is a bit more complicated than just 'concentration', although
it includes this idea. The higher the concentration of dissolved solutes,
the lower the 'concentration' of water and the lower the water potential.
These solutes may be one or many things, so water potential is a function
of total solute concentration. So, water will osmose across a membrane from
an area of low solute concentration (high water potential) to an area of
high solute concentration (low water potential), or in other words, from
a dilute solution to a concentrated solution. Another component of water
potential is water pressure: water will also cross a membrane due to pressure
exerted by an outside force or the force of its own mass responding to gravity.
So water can be pushed across a membrane.
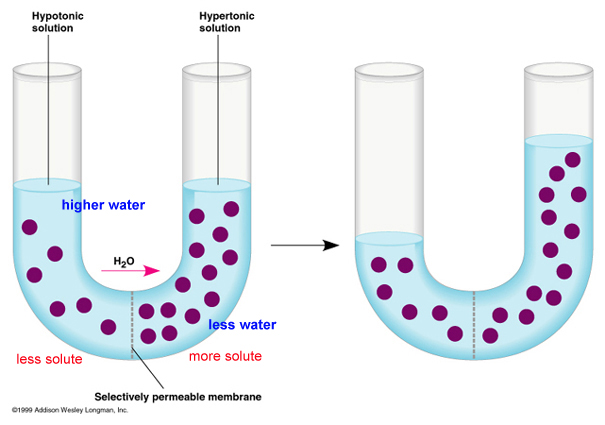
Consider the figure, below. The
purple circles are dissolved solutes. SO! In the first figure at left, total
solute concentration is HIGH on the right side of the u-tube, so water potential
is LOW . Water will move across the membrane from the dilute solution on
the left (high water potential) to the concentrated solution on the right
(low water potential), and the water level will rise (figure on the right).
Eventually, in this rigid vertical system, the tendancy of the water to
move left to right in response to solute concentration is balanced by the
tendancy of water to move right to left - "leaking" back across
the membrane - in response to the greater water pressure. An equilibrium
is reached where there is no NET flow. In a rigid plant cell (with a cell
wall), the influx of water creates "hydrostatic pressure" and
makes the cell rigid or 'turgid'. The loss of water (from evapotranspiration
or osmosis out of the cells to a saltier environment) reduces this turgidity
- or 'turgor pressure' - and the plant tissue wilts (gets 'floppy'). In
an animal cell bounded only by a thin membrane, the influx of water can
create a pressure large enough to rupture the cell. We will deal with these
concepts more in lab.
 b.
Faciliated Diffusion:
Large polar molecules cannot diffuse across the lipid bilayer. However, they
can cross the membrane 'passively' from high to low concentration, through
integrated proteins channels. Some of these channels are rather unspecialized
'tubes', while other channels are rather specific and will only permit the
transport of certain classes of molecules. In any case, this is the way that
large polar molecules cross the membrane in response to their concentration
gradient (high to low). video.
b.
Faciliated Diffusion:
Large polar molecules cannot diffuse across the lipid bilayer. However, they
can cross the membrane 'passively' from high to low concentration, through
integrated proteins channels. Some of these channels are rather unspecialized
'tubes', while other channels are rather specific and will only permit the
transport of certain classes of molecules. In any case, this is the way that
large polar molecules cross the membrane in response to their concentration
gradient (high to low). video.
c. Active
Transport: If material
only crossed the membrane by some form of diffusion, then the cell's cytoplasm
would come to be very similar to the surrounding environment. They cell would
be unable to raise or lower the concentration of material above the concentration
in the environment. In order for cells to be differnt from the environment
(in the concetration of some stuff), another mechanism is needed. This is
'active transport'. In active transport, a cell uses energy to 'pump' material
across the membrane - against the concentration gradient (from low to high).
So, although sugars might be in higher concetration within the cell than outside
it, and although sugars may be "leaking" from the cell by diffusion,
the cell can pump sugar against the concentration gradient and accumulate
it in the cell. Likewise, a cell can pump toxins or waste OUT of the cell,
even if the concentration outside of the cell is already high. THIS TAKES
ENERGY - LIKE ROLLING A BALL UPHILL, AGAINST THE 'GRADIENT' OR SLOPE. An important
active transport mechanism is the "sodium-potassium" pump. In this
process, both ions are pumped against their concentration gradient, using
energy (breaking ATP) to change the conformation of the transport protein.
video
3. Metabolism: Some of the proteins
associated with the membrane are enzymes that catalyze reactions. By positioning
enzymes next to one another that catalyze sequential reactions in a process,
the process can run much more efficiently.
4. Signal transduction: Proteins
can also be involved with 'perception'. When a membrane protein binds a compound
in the environment, it may change shape. This shape change may release a subunit
inside the cell that binds to something else and initiates a cellular response.
So the cell perceives a stimulus in the environment and responds - all at the
chemical level. The percetion of a signal can stimulate the activation or inactivation
of a gene - and thus affect the proteins that a cell produces and the cell's
basic physiology. video
5. Cell-cell recognition: Surface
proteins and carbohydrates give a cell a chemical 'signature'. This is critical
in the immune system, where cells are identified as "self" or "foreign"
based on these surface antigens.
6. Cell binding: In many tissues
(but not all, such as blood), the cells are bound together; sometimes quite
tightly. This cell-cell binding usually involves proteins that interlock and
bind together.
7. Attachment of the cytoskeleton:
a cell is not a "baggie" with organelles floating around in the cytoplasm.
Most organelles (like mitochondria and chromosomes) are bound to cytoskeletal
fibers that hold them in position. The cytoskeleton is also responsible for
changin the cells' shape.
IV.
Harvesting Energy: Cellular Respiration
Overview:
In this lecture, 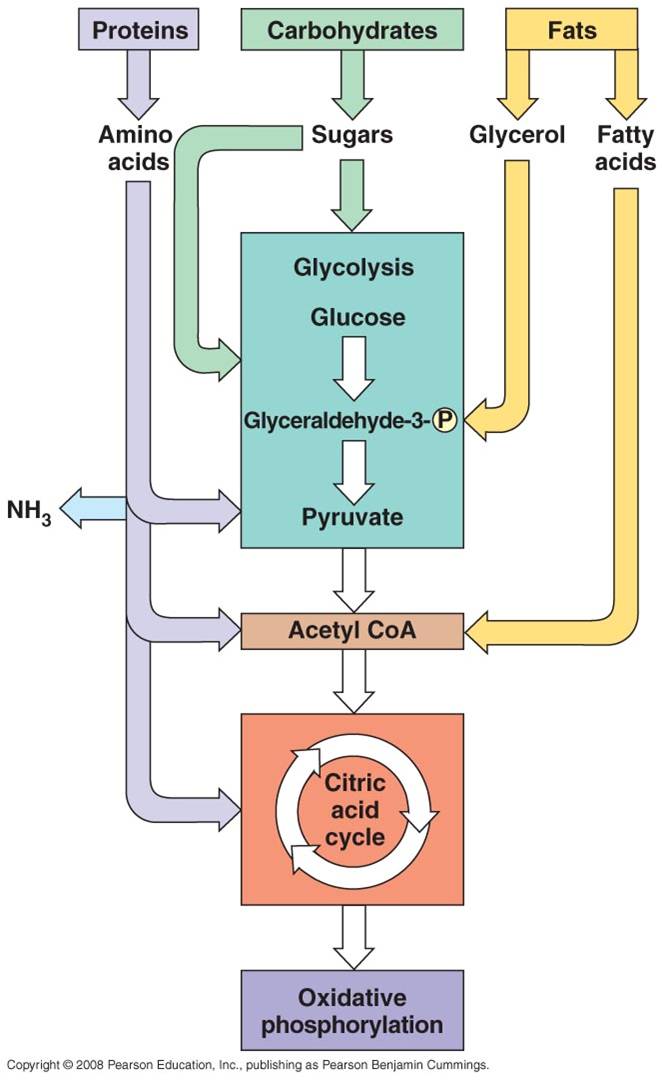 we will examine the energy harvesting
reactions that ALL living cells perform: Cellular Respiration. In other words, "what happens to the food you eat?" And, "what happens to the oxygen you breathe in?" All
living cells - eubacteria, archaea, protists, fungi, plants, and animals - can
harvest the energy contained in the chemical bonds of complex organic molecules. By breaking the covalent bonds between carbon atoms in these molecules, energy
is released. The energy released by these reactions must be trapped in other bonds or used to do work; otherwise it is lost as heat. So, cells perform coupled reactions to TRAP the energy. One reaction breaks down bonds in food, and the energy released is TRAPPED in new bods formed between
ADP and P --> making ATP. As such, some of the energy
in the covalent bonds of the initial organic molecules is transformed into chemical
energy in bonds of ATP. Carbon-carbon bonds are very strong and stable; enzymes can't break these bonds. These bonds are like a $100 bill--you can't use it everywhere...some stores won't take them. The energy must be converted to a 'lower denominationa; form' that can be used by all enzymes ('stores') in the cell. So, when the 5 carbon-carbon bonds in a glucose are broken, 36-38 bonds are made between ADP and P. And so, each of these bonds in ATP is MUCH weaker (contains less energy)... and most of the energy released by the breaking of the carbon-carbon bonds is lost as heat! Energy in this form is now available to all of the enzymes
in the cell, for catalyzing their own reactions (chemical energy) or doing work
like muscular contraction (mechanical energy) or pumping ions across a membrane
against their concentration gradient (active transport).
we will examine the energy harvesting
reactions that ALL living cells perform: Cellular Respiration. In other words, "what happens to the food you eat?" And, "what happens to the oxygen you breathe in?" All
living cells - eubacteria, archaea, protists, fungi, plants, and animals - can
harvest the energy contained in the chemical bonds of complex organic molecules. By breaking the covalent bonds between carbon atoms in these molecules, energy
is released. The energy released by these reactions must be trapped in other bonds or used to do work; otherwise it is lost as heat. So, cells perform coupled reactions to TRAP the energy. One reaction breaks down bonds in food, and the energy released is TRAPPED in new bods formed between
ADP and P --> making ATP. As such, some of the energy
in the covalent bonds of the initial organic molecules is transformed into chemical
energy in bonds of ATP. Carbon-carbon bonds are very strong and stable; enzymes can't break these bonds. These bonds are like a $100 bill--you can't use it everywhere...some stores won't take them. The energy must be converted to a 'lower denominationa; form' that can be used by all enzymes ('stores') in the cell. So, when the 5 carbon-carbon bonds in a glucose are broken, 36-38 bonds are made between ADP and P. And so, each of these bonds in ATP is MUCH weaker (contains less energy)... and most of the energy released by the breaking of the carbon-carbon bonds is lost as heat! Energy in this form is now available to all of the enzymes
in the cell, for catalyzing their own reactions (chemical energy) or doing work
like muscular contraction (mechanical energy) or pumping ions across a membrane
against their concentration gradient (active transport).
All four classes of biological molecules
(carbo's, fats, proteins, and nucleic acids) are broken down for energy harvest (they all contain lots of carbon-carbon bonds).
The process of carbohydrate metabolism, however, is the central process.
Fats, proteins, and nucleic acids are broken into their monomers, these are
modified, and then these products can be shunted into the carbohydrate digestion
process. So, although we will focus on carbohydrate metabolism - and glucose
metabolism in particular - you should appreciate that all other polymers can
be broken down for energy harvest. And respiration not only harvests energy
- respiration also provides the monomers needed by the cell to build its own
biomolecules. So, when you digest protein, energy is harvested and the separated
amino acids can be used by your cells to make your DNA-specified proteins. This
is why a balanced diet is important - digestion of varied complex organic molecules
provides the different monomers and other essential vitamins and minerals (often
used as cofactors in reactions) that your cells require.
The metabolism of glucose can accur
in the presence of absence of oxygen. The first step is glycolysis, in which
the six-carbon sugar is split into 2 C3 molecules of pyruvate. The breaking
of this bond releases a small amount of energy. In the absence of oxygen, fermentation
occurs. The primary function of this "anaerobic" respiration is to
recyclce some chemicals needed to keep glycolysis going. So, anaerobic respiration,
including glycolysis and fermentation, breaks only a couple bonds and produces
only a small amount of energy. In the presence of oxygen, the pyruvates can
be completely oxidized. The C3 molecules are completely broken down into 3 one-carbon
molecules of carbon dioxide. The complete breakdown of the the pyruvates releases
much more energy. This is probably why aerobic organisms have come to dominate
the planet - they harvest more energy from the food they consume, and can use
this energy to survive and reproduce more effectively.
1. Glycolysis
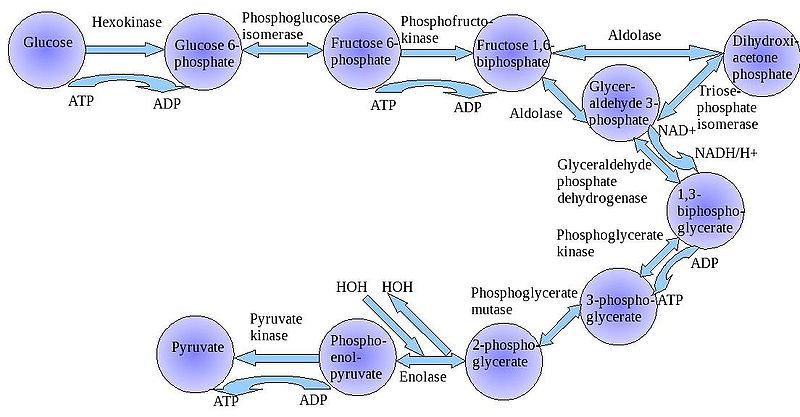 The
"splitting of glucose" (glyco-lysis) is probably an ancient metabolic
reaction; it is performed in the cytoplasm of ALL living cells from prokaryotes
to eukaryotes, and cells can perform this reaction in the presence OR absence
of oxygen gas. So, it seems likely that this was an important energy harvesting
reaction for ancient cells that lived before ~2 bya - before oxygen became abundant
in the oceans and atmosphere. As you can see in the flowchart, glycolysis is
not ONE reaction - it is a series of reactions catalyzed by a variety of enzymes.
The
"splitting of glucose" (glyco-lysis) is probably an ancient metabolic
reaction; it is performed in the cytoplasm of ALL living cells from prokaryotes
to eukaryotes, and cells can perform this reaction in the presence OR absence
of oxygen gas. So, it seems likely that this was an important energy harvesting
reaction for ancient cells that lived before ~2 bya - before oxygen became abundant
in the oceans and atmosphere. As you can see in the flowchart, glycolysis is
not ONE reaction - it is a series of reactions catalyzed by a variety of enzymes.
video
2. Aerobic Respiration
Oxygen is a very reactive gas - it
oxidizes things - stripping electrons from other molecules and breaking bonds.
Combustion is an oxidative process, and it can occur spontaneously, without
an ignition source. So, if combustible material heats up above its ignition
temperature, and if a strong oxidative agent like oxygen is present, it will
ignite. Gasoline is a long hydrocarbon polymer. When raised above it's ignition
point, it will combust. The gasoline will be oxidized to CO2 and
H2O - and the breaking of the carbon-carbon bonds that occurs during
this process will release energy. A gallon of gasoline contains ALOT of bonds
and ALOT of energy; and if it is released all at once, the energy is difficult
to control or use - you get an uncontrolled explosion. In a car's internal combustion
engine, very small amounts of gasoline are squrted into the cylinder heads in sequence, ignited by spark plugs in sequence,
causing a little explosion in each cylinder that pushes the piston in that cylinder down, turning
the crankshaft that turns axle that turns the wheels of the car. In a diesel engine, there are
no spark plugs and no spark; the fuel ignites when the temperature exceeds its
ignition point when placed under high pressure when the piston rises. By controling
the reaction, by oxidizing just a little at a time, the energy released can
be used to do work.
a. Overall Process: - Pyruvates from glycolysis are broken down
into carbon dioxide.
- Energy that is released from
the complete breadown of the C-C bonds is used to make bonds in ATP (38).
- When bonds are broken, electrons
are released.
- Ultimately, the electrons
are passed to Oxygen O--, which then binds two hydrogen ions to balance charge
(forming water).
- Aerobic respiration is a
more complete breakdown of glucose, so it yields more ATP than glycolysis, alone
- In eukaryotes, this occurs
in a three step process in the mitochondria of cells. In the gateway step, one carbon is broken off each pyruvate s carbon dioxide. In the second step, the remaining 2-carbon molecules are split into 2 co2. Through these reactins, most of the energy has not been immdiately trapped in ATP; it has been trapped in NADH and FADH (other energy currency molecules). In the thrid step, NADH an FADH give up their high eneergy electrons, which are passed in the electron transport chain and their energy is used to make ATP. After they have given up their energy, they are absorbed by oxygen, and H+, making water. SO, the oxygen you breathe in is converted to water; you don't breathe it out directly as CO2!!!
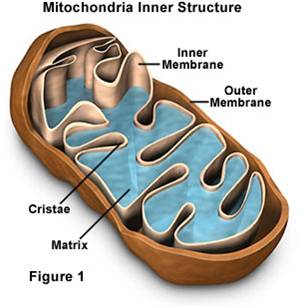 Mitochondria have a double membrane
system like bacteria and chloroplasts, with an intermembrane space and matrix
within inner membrane.
Mitochondria have a double membrane
system like bacteria and chloroplasts, with an intermembrane space and matrix
within inner membrane.
- They have their own DNA, and they
replicate themselves by fission - they aren't 'made' by the cell.
- Given these observations, Lynn
Margulis hypothesized that these similarities were due to common ancestry, rather
than common environment. She raised this hypothesis as the endosymbiotic
hypothesis of eukaryote evolution, hypothesizing that eukaryotes acquired their
organelles by engulfing free-living bacteria and, rather than digesting them,
simply engulfed them and consumed their products (in this case the ATP that
the bacteria produce. The relationship is called symbiotic, because Margulis
hypothesized that the bacteria would also benefit by being in a stable environment
where the concentration of glucose was high (inside the cell).
- The most direct test of a hypothesis
of relatedness is DNA similarity. DNA only comes from parents, so similarities
imply a common source. When these tests were performed in the 1970's, her hypothesis
was confirmed. Additional tests with choloplasts and basal bodies (other
organielles in eukaryotes) also showed strong patterns of relatedness with free-living
bacteria. As such, we now refer to this tested model as the Endosymbiotic
Theory.
V. Harvesting Energy: Photosynthesis
Overview:
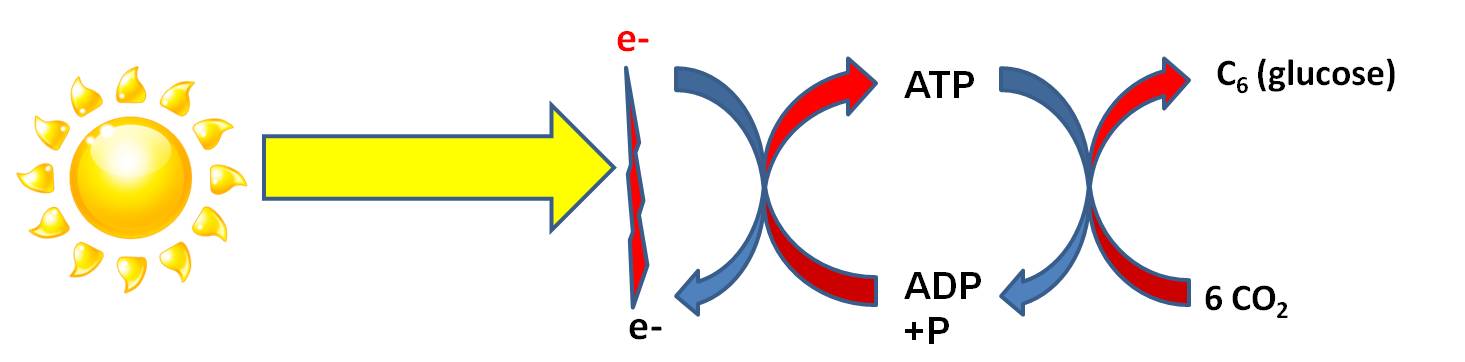 Although all organisms (plants, animals, fungi, protists, bacteria) can harvest energy by breaking down organic molecules (Cellular Respiration), some have evolved a mechanism for transforming radiant energy in chemical bond energy. Photosynthesis
is that process of energy transformation. Again, although energy can neither be
created nor destroyed, it can be transformed. In the "Light Dependent Reaction"
radiant energy ('carried' by photons in light) is transformed into chemical
energy ('carried' by electrons). It requires an electron DONOR to provide electrons
that will 'carry' this energy. The energy 'carried' by this electron is used
to form a bond between ADP and P, creating ATP. Through this transfer, the electron
loses this energy. As we have discussed before, the phosphate bonds in ATP are
easily made and easily broken - that's why energy in this form of chemical bond
can be 'used' by all enzymes in the cell. However, ATP is readily hydrolyzed
in water...so it is difficult for a cell to build up a large amount of ATP before
it 'dissolves' to ADP and P again. To store large amounts of energy for a longer
time, the energy in ATP can be converted to a more stable molecule. In most
photosynthetic organisms, the catabolism of ATP is coupled to anabolic reactions
that bind carbon dioxide molecules together into stable molecules of glucose,
for longer term E storage. This also provides the cell with organic carbon that
it can use to make the other biologically important molecules. These are the
"Light Independent Reactions" of photosynthesis.
Although all organisms (plants, animals, fungi, protists, bacteria) can harvest energy by breaking down organic molecules (Cellular Respiration), some have evolved a mechanism for transforming radiant energy in chemical bond energy. Photosynthesis
is that process of energy transformation. Again, although energy can neither be
created nor destroyed, it can be transformed. In the "Light Dependent Reaction"
radiant energy ('carried' by photons in light) is transformed into chemical
energy ('carried' by electrons). It requires an electron DONOR to provide electrons
that will 'carry' this energy. The energy 'carried' by this electron is used
to form a bond between ADP and P, creating ATP. Through this transfer, the electron
loses this energy. As we have discussed before, the phosphate bonds in ATP are
easily made and easily broken - that's why energy in this form of chemical bond
can be 'used' by all enzymes in the cell. However, ATP is readily hydrolyzed
in water...so it is difficult for a cell to build up a large amount of ATP before
it 'dissolves' to ADP and P again. To store large amounts of energy for a longer
time, the energy in ATP can be converted to a more stable molecule. In most
photosynthetic organisms, the catabolism of ATP is coupled to anabolic reactions
that bind carbon dioxide molecules together into stable molecules of glucose,
for longer term E storage. This also provides the cell with organic carbon that
it can use to make the other biologically important molecules. These are the
"Light Independent Reactions" of photosynthesis.
 When
we think of photosynthesis, most of us think "plants". This is generally
correct, but very incomplete. First, there are some plants like Indian Pipe
(Monotropa uniflora) that do not photosynthesize. Although they evolved
from photosynthetic ancestors, they have adopted a parasitic lifestyle and no
longer harvest their own energy from sunlight. In addition, there are photosynthetic
protists (algae and Euglenozoans), and photosynthetic archaeans and eubacteria.
In fact, there are several animals that harbor photosynthetic symbionts, too.
Many corals (corals are animals) ingest algal cells and distribute them to their
tentacles. The algae photosynthesize, and excess sugars are passed to the coral
animal. These symbiotic algae give corals their spectacular colors. When stressed
by water polution or high water temperatures, the corals release their symbionts
and lose their color ("a phenomenon called "coral bleaching").
Long periods without their symbionts results in coral death.
When
we think of photosynthesis, most of us think "plants". This is generally
correct, but very incomplete. First, there are some plants like Indian Pipe
(Monotropa uniflora) that do not photosynthesize. Although they evolved
from photosynthetic ancestors, they have adopted a parasitic lifestyle and no
longer harvest their own energy from sunlight. In addition, there are photosynthetic
protists (algae and Euglenozoans), and photosynthetic archaeans and eubacteria.
In fact, there are several animals that harbor photosynthetic symbionts, too.
Many corals (corals are animals) ingest algal cells and distribute them to their
tentacles. The algae photosynthesize, and excess sugars are passed to the coral
animal. These symbiotic algae give corals their spectacular colors. When stressed
by water polution or high water temperatures, the corals release their symbionts
and lose their color ("a phenomenon called "coral bleaching").
Long periods without their symbionts results in coral death.
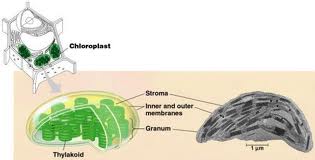 Photosynthesis
in prokaryotes occurs on the double-membrane system of these organisms. In eukaryotes,
photosynthesis occurs in organelles called chloroplasts. Chloroplasts have a
bacteria-like double membrane, and they have their own DNA. This DNA is more
similar in most respects to the DNA in free-living bacteria than to the DNA
in the nucleus of the eukaryotic cells they 'inhabit'. For these reasons, most
scientists accept the 'endosymbiotic theory' of chloroplast origins. This theory
states that chloroplasts in the cells of photosynthetic eukaryotes are descendants
of free-living photosynthetic bacteria. At some point in the early evolution
of protists, these photosynthetic bacteria were engulfed by not digested. Rather,
the host cells fed on the excess sugars produced by the internalized bacteria.
Eventually, as the result of gene exchange between the host and proto-chloroplasts,
the eukaryotic host and the prokaryotic symbiont became dependent on one another.
But chloroplasts can still live outside of cells for several days. Plants, evolving
from green algae ancestors, inherited these bacteria-like chloroplasts, too.
Photosynthesis
in prokaryotes occurs on the double-membrane system of these organisms. In eukaryotes,
photosynthesis occurs in organelles called chloroplasts. Chloroplasts have a
bacteria-like double membrane, and they have their own DNA. This DNA is more
similar in most respects to the DNA in free-living bacteria than to the DNA
in the nucleus of the eukaryotic cells they 'inhabit'. For these reasons, most
scientists accept the 'endosymbiotic theory' of chloroplast origins. This theory
states that chloroplasts in the cells of photosynthetic eukaryotes are descendants
of free-living photosynthetic bacteria. At some point in the early evolution
of protists, these photosynthetic bacteria were engulfed by not digested. Rather,
the host cells fed on the excess sugars produced by the internalized bacteria.
Eventually, as the result of gene exchange between the host and proto-chloroplasts,
the eukaryotic host and the prokaryotic symbiont became dependent on one another.
But chloroplasts can still live outside of cells for several days. Plants, evolving
from green algae ancestors, inherited these bacteria-like chloroplasts, too.
 Photosynthesis
is a critically important process in the evolution and diversity of life. Prior
to the evolution of photosynthesis, life was dependent on absorbing spontaneously
generated organic molecules, or preying on other cells. Neither of these sources
of energy was probably all that common and easy to find. Evolving the ability
to use sunlight as an energy source, which IS abundant and IS easy to find,
meant that life could grow, prosper, and radiate dramatically - almost anywhere
there was a light source. Indeed, it looks like photosynthesis evolved very
early in the history of life; the earliest fossils (stromatolites and filamentous
microfossils dating to ~3.5 by) look very similar to photosynthetic bacteria
that are alive today. When photosynthetic organisms became abundant, they provided
a food supply for a wider variety of heterotrophic cells. Heterotrophs could
then live anywhere phototrophs lived; they were not limited to those rare places
where biological molecules were forming spontaneously. So, complex bacterial
food webs evolved. These early photosynthetic organisms used a primitive form
of photosynthesis that did not produce oxygen as a waste product. So, even though
they flourished for a billion years, no oxygen was added to the atmosphere.
About 2.0 billion years ago, a 'modern' type of photosynthesis evolved that
used water as the electron donor and produced oxygen gas as a waste product.
The production of oxygen gas transformed the oceans (precipitating iron), and
eventually changed the atmosphere, as well. Although oxygen was probably a highly
toxic gas at first (because it is so reactive), life eventually evolved to tolerate
it and then to USE it in oxidative respiration. The evolution of aerobic respiration
allowed for more energy to be harvested from the catabolism of complex organic
molecules, and may have allowed for the evolution of more energy-demanding eukaryotes
and multicellular organisms. As you know, almost all food webs are ultimately
dependent on the photosynthetic organisms at the base of the "food chain"
(hydrothermal vent communities are a possible exception). We use this energy
to stick amino acids together to make our proteins, etc. Even the gas and oil
that powers our industrial societies was initally stored as glucose produced
by photosynthesis. Coal, gas, and oil are just fossilized plants - and we "burn"
that energy millions of years after it was converted from sunlight. We are powering
our societies with sunlight that hit the Earth millions of years ago. But not
only are you (and every other heterotroph) energetically dependant on photosynthetic
organisms for food, you are also indebted to them for changing the planet and
stimulating the evolution of eukaryotic and multicellular life. In short, there
are few processes more important to the history and current function of living
systems (and our petroleum-based economy) than photosynthesis.
Photosynthesis
is a critically important process in the evolution and diversity of life. Prior
to the evolution of photosynthesis, life was dependent on absorbing spontaneously
generated organic molecules, or preying on other cells. Neither of these sources
of energy was probably all that common and easy to find. Evolving the ability
to use sunlight as an energy source, which IS abundant and IS easy to find,
meant that life could grow, prosper, and radiate dramatically - almost anywhere
there was a light source. Indeed, it looks like photosynthesis evolved very
early in the history of life; the earliest fossils (stromatolites and filamentous
microfossils dating to ~3.5 by) look very similar to photosynthetic bacteria
that are alive today. When photosynthetic organisms became abundant, they provided
a food supply for a wider variety of heterotrophic cells. Heterotrophs could
then live anywhere phototrophs lived; they were not limited to those rare places
where biological molecules were forming spontaneously. So, complex bacterial
food webs evolved. These early photosynthetic organisms used a primitive form
of photosynthesis that did not produce oxygen as a waste product. So, even though
they flourished for a billion years, no oxygen was added to the atmosphere.
About 2.0 billion years ago, a 'modern' type of photosynthesis evolved that
used water as the electron donor and produced oxygen gas as a waste product.
The production of oxygen gas transformed the oceans (precipitating iron), and
eventually changed the atmosphere, as well. Although oxygen was probably a highly
toxic gas at first (because it is so reactive), life eventually evolved to tolerate
it and then to USE it in oxidative respiration. The evolution of aerobic respiration
allowed for more energy to be harvested from the catabolism of complex organic
molecules, and may have allowed for the evolution of more energy-demanding eukaryotes
and multicellular organisms. As you know, almost all food webs are ultimately
dependent on the photosynthetic organisms at the base of the "food chain"
(hydrothermal vent communities are a possible exception). We use this energy
to stick amino acids together to make our proteins, etc. Even the gas and oil
that powers our industrial societies was initally stored as glucose produced
by photosynthesis. Coal, gas, and oil are just fossilized plants - and we "burn"
that energy millions of years after it was converted from sunlight. We are powering
our societies with sunlight that hit the Earth millions of years ago. But not
only are you (and every other heterotroph) energetically dependant on photosynthetic
organisms for food, you are also indebted to them for changing the planet and
stimulating the evolution of eukaryotic and multicellular life. In short, there
are few processes more important to the history and current function of living
systems (and our petroleum-based economy) than photosynthesis.
A. Step 1: The Light Dependent Reaction
AGAIN, the purpose of the light-dependent reaction is to convert radiant energy
to chemical energy. Obviously, light must be present; so this reaction "depends"
on sunlight.
- In photosynthetic Eukaryotes(photosynthetic protists and plants), these reactions
occur on the inner membrane of the Chloroplast - a specific membrane-bound organelle
very much like a bacterium within the larger eukaryotic cell. Indeed, as described
above, eukaryotic chloroplasts are probably the deescendants of free-living
cyanobacteria - with whom they share basic membrane structure and DNA similarity.
- In cyanobacteria and chloroplasts, there
are two types of reaction centers called "photosystems". The second
photosystem (PSII) has a lower electronegativity than the first, so it can exert
a 'stronger' pull and can strip electrons from WATER (which holds the electrons
more strongly than H2S does.) The splitting of water releases oxygen
gas as a waste product, so this type of photosynthesis is also called "oxygenic
photosynthesis".
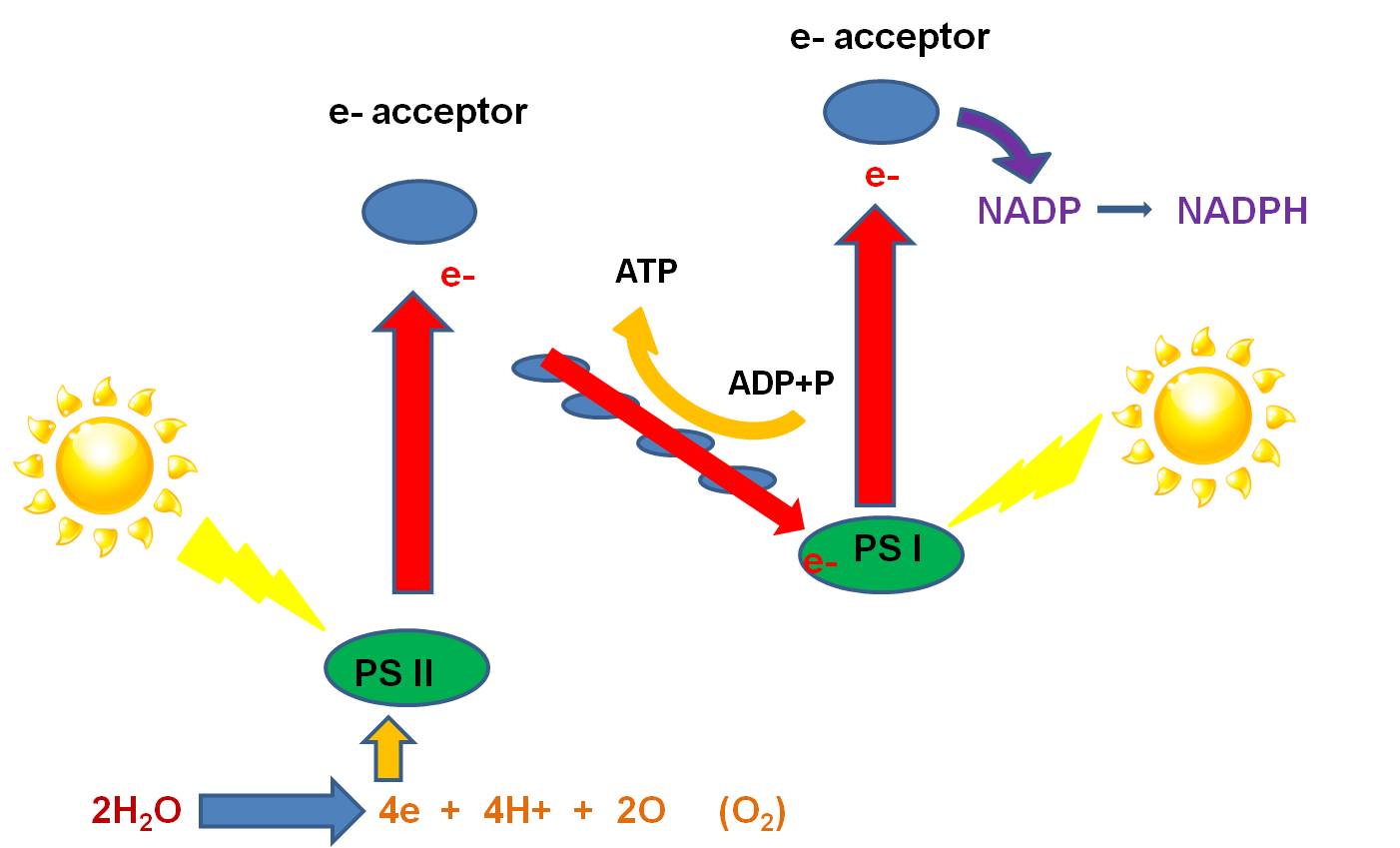 - Here's how it works: Light strikes the phosystems nested in the inner membrane
(called the 'thylakoid' membrane in chloroplasts). An electron in each photosystem
is excited and lost from the Mg in the chlorophyll molecule. The electrons are
accepted by particular electron acceptor molecules. The electron lost from PS
I is ultimately passed to NADP, which accepts a H+ to balance the charge, making
the high energy molecule, NADPH. The electron lost from PSII is passed to an
electron acceptor, and then to molecules in the electron transport chain. As
the electron is passed down the chain, ATP is produced by chemiosmosis (as described
above). When this electron has lost it's energy, it replaces the electron lost
from PS I. So, PS I is all set, and need not strip electrons from an electron
donor. However, PS II has lost an electron, and must replace this electron for
photosynthesis to continue. PSII strips electrons from H2O. Water
is split into oxygen, 2 H+, and 2 electrons. The electrons are passed to the
cholorophyll in PS II, excited by light, and energized. The oxygen reacts with
another oxygen atom to produce oxygen gas, which is released as a waste product.
The propose of photosynthesis is not "to produce oxygen". The purpose
of the light reaction of photosynthesis is to transform radiant energy into
chemcial energy, and produce ATP and NADPH. The two molecules, ATP and NADPH,
are the useful products. Again, oxygen gas is produced as a waste product when
electrons are stripped from water. The presence of oxygen in the oceans 2.5-2
billion years ago, indicated by the presence of sedimentary deposits with oxidized
iron (banded iron formations), indicates the evolution of this more advanced
type of photosynthesis that evolved in ancient photosynthetic bacteria.
- Here's how it works: Light strikes the phosystems nested in the inner membrane
(called the 'thylakoid' membrane in chloroplasts). An electron in each photosystem
is excited and lost from the Mg in the chlorophyll molecule. The electrons are
accepted by particular electron acceptor molecules. The electron lost from PS
I is ultimately passed to NADP, which accepts a H+ to balance the charge, making
the high energy molecule, NADPH. The electron lost from PSII is passed to an
electron acceptor, and then to molecules in the electron transport chain. As
the electron is passed down the chain, ATP is produced by chemiosmosis (as described
above). When this electron has lost it's energy, it replaces the electron lost
from PS I. So, PS I is all set, and need not strip electrons from an electron
donor. However, PS II has lost an electron, and must replace this electron for
photosynthesis to continue. PSII strips electrons from H2O. Water
is split into oxygen, 2 H+, and 2 electrons. The electrons are passed to the
cholorophyll in PS II, excited by light, and energized. The oxygen reacts with
another oxygen atom to produce oxygen gas, which is released as a waste product.
The propose of photosynthesis is not "to produce oxygen". The purpose
of the light reaction of photosynthesis is to transform radiant energy into
chemcial energy, and produce ATP and NADPH. The two molecules, ATP and NADPH,
are the useful products. Again, oxygen gas is produced as a waste product when
electrons are stripped from water. The presence of oxygen in the oceans 2.5-2
billion years ago, indicated by the presence of sedimentary deposits with oxidized
iron (banded iron formations), indicates the evolution of this more advanced
type of photosynthesis that evolved in ancient photosynthetic bacteria.
B. Step
2: The Light Independent Reactions:
The purpose of the "Light Independent
Reactions" is to convert the chemical energy in fragile ATP and NADPH molecules
into a more stable energy form by building covalent bonds between carbon atoms
to make glucose. In prokaryotes, these reactions occur in the cytoplasm of the
cell; in eukaryotes, these reactions occur in the stroma - or cytoplasm - of
the chloroplasts. It is important to appreciate that organisms using both primitive
and advanced light reactions perform the light independent reactions.
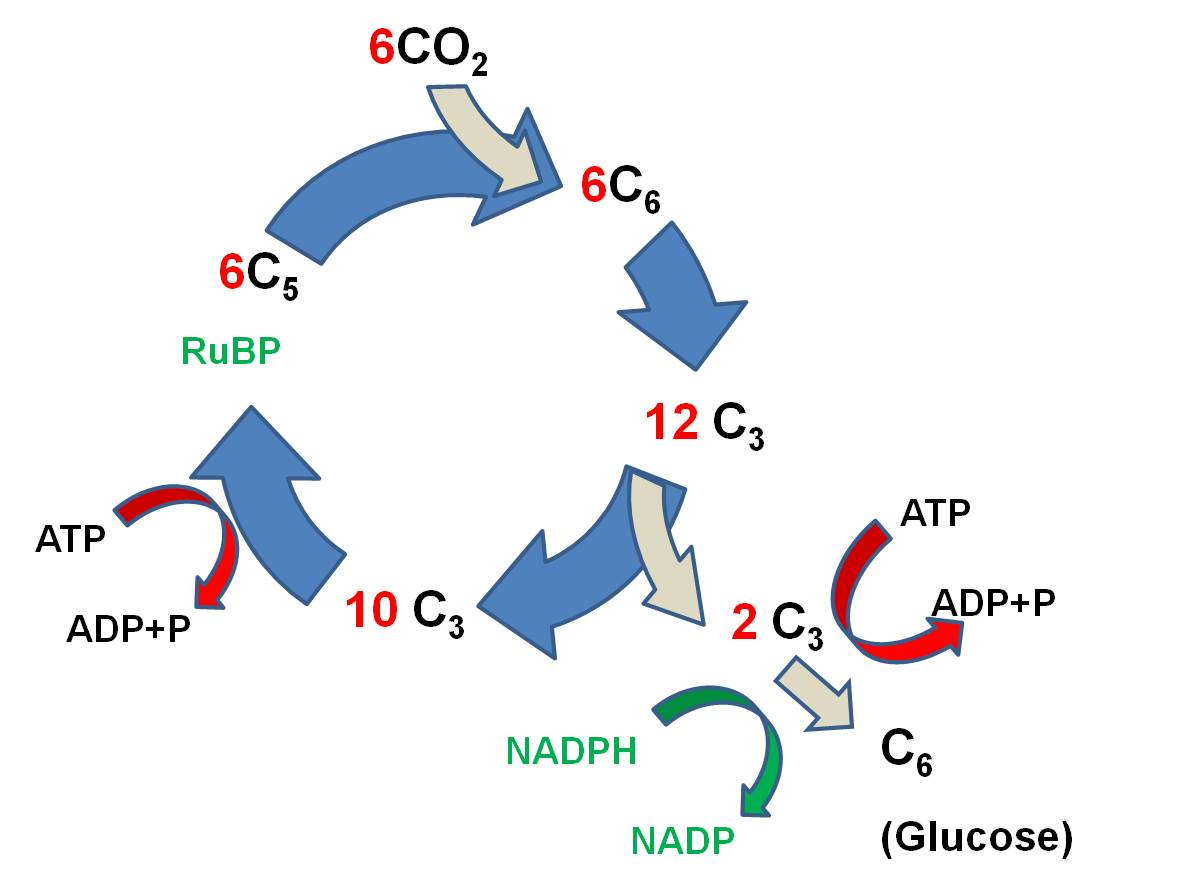 The
primary reaction is called the Calvin-Benson
Cycle, and it works like this:
The
primary reaction is called the Calvin-Benson
Cycle, and it works like this:
- 6 CO2 molecules bind
to 6 C5 molecules of Ribulose Biphosphate (RuBP), making 6 C6 molecules. (ATP is broken and the energy that is released is used to link
CO2 to RUBP).
- These energized C6 molecules
are unstable; the split into 12 C3 molecules. So, since the first
stable product is a C3 molecule, this type of reaction is called the C3 pathway.
- 2 C3 molecules are used
to form 1 glucose (C6) molecule. More ATP is used, and NADPH is used,
too, and H is transferred to put the 'hydrogen' in 'carbohydrate'.
- the 10 remaining C3 molecules (30 C total) are rearranged, using ATP and NADPH, and 6 C5 molecules are generated (30 C total).
The reaction can be summarized like
this: Six CO2 molecules are used to make one molecule of glucose. Six RuBP
molecules are involved, and are recycled through the process. The ATP and NADPH
formed in the light reaction are used to power this reaction; the energy in
these molecules is used top make bonds between the CO2, and the H
from NADPH is used to reduce the CO2 to form glucose (C6H12O6).
As such, the radiant energy initially trapped in chemical bonds in ATP and NADPH
is transferred to form bonds between carbon atoms in glucose. The energy intially
trapped in fragile molecules has been stored in a more stable form.
When cells build glucose from CO2,
they have not only stored energy in a stable form - they have also harvested
carbon from the environment and transformed it into a usable organic molecule.
Since all biologically important molecules (except water) are carbon-based organic
molecules, all life forms needs a source of carbon to build amino acids, nucleotides,
sugars, and lipids. "Heterotrophs" get organic carbon in the 'food'
they eat. "Autotrophs" get their carbon through the light independent
reaction, which also stores energy.
The first group of bacteria discussed
above - the green non-sulphur bacteria and purple non-sulphur bacteria - perform
the Light Dependent Reaction and make ATP using sunlight, but they do not perform
the light indepedent reactions. So, they do not absorb CO2 to make their organic
molecules. Instead, they must consume organic molecules to acquire their carbon.
These organisms are "photoheterotrophs". They may represent the first
step in the evolution of photosynthesis: the evolution of light-trapping reactions
by heterotrophic cells. They use cyclic phosphorylation to make ATP in the presence
of light, but they use organic molecules as electron donors.
VI. Using Energy: Protein Synthesis
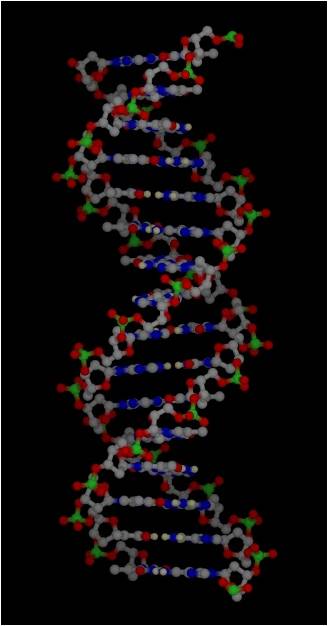 Much of the energy harvested by a cell is used to make proteins. However, in order to understand protein synthesis, we must first describe the structure of DNA. DNA is the 'recipe' for proteins, so we need to take a little detour on our jounrey of how a cell works to describe the structure of DNA and chromosomes. DNA
is the genetic material in all forms of life (eubacteria, archaea, protists,
plants, fungi, and animals). Those quasi-living viruses vary in their genetic
material. Some have double-stranded DNA (ds-DNA) like living systems, while
others have ss-DNA, ss-RNA, and ds-RNA. RNA performs a wide array of functions
in living systems. Many of these functions have only been discovered in the
last few years.
Much of the energy harvested by a cell is used to make proteins. However, in order to understand protein synthesis, we must first describe the structure of DNA. DNA is the 'recipe' for proteins, so we need to take a little detour on our jounrey of how a cell works to describe the structure of DNA and chromosomes. DNA
is the genetic material in all forms of life (eubacteria, archaea, protists,
plants, fungi, and animals). Those quasi-living viruses vary in their genetic
material. Some have double-stranded DNA (ds-DNA) like living systems, while
others have ss-DNA, ss-RNA, and ds-RNA. RNA performs a wide array of functions
in living systems. Many of these functions have only been discovered in the
last few years.
 A.
DNA and RNA Structure
A.
DNA and RNA Structure
DNA (deoxyribonucleic acid) and RNA
(ribonucleic acid) are nucleic acids - polymers consisting of a linear
sequence of linked nucleotide monomers. We will describe the structure of the
monomers first, and then describe how they are linked into linear polymers.
Finally, we will describe the double-stranded structure of ds-DNA.
1. The monomers
are "nucleotides"
three components:
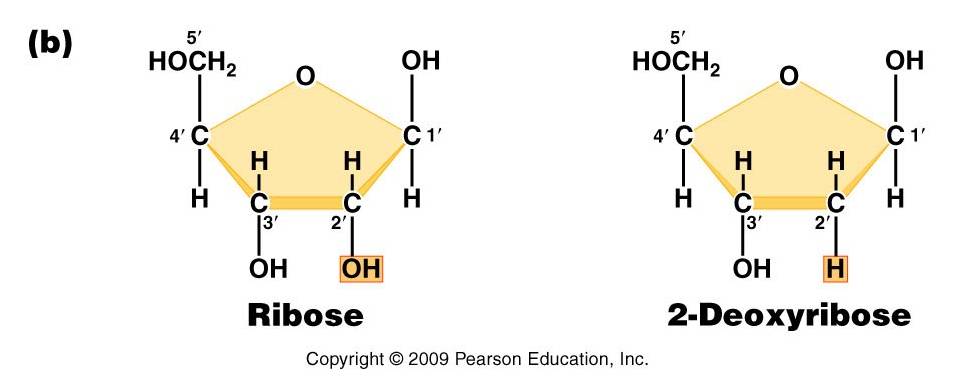
 -
Pentose (5 carbon) sugar: either ribose (RNA) or deoxyribose
(DNA). The carbons are numbered clockwise. The difference between the sugars
is that ribose has an -OH group on the 2' carbon, whereas deoxyriboes has
only 2 H groups and thus is "deoxygenated" relative to ribose. BOTH
sugars have an -OH group on the 3' carbon, which will be involved in binding.
The 5' carbon is a sidegroup off the ring.
-
Pentose (5 carbon) sugar: either ribose (RNA) or deoxyribose
(DNA). The carbons are numbered clockwise. The difference between the sugars
is that ribose has an -OH group on the 2' carbon, whereas deoxyriboes has
only 2 H groups and thus is "deoxygenated" relative to ribose. BOTH
sugars have an -OH group on the 3' carbon, which will be involved in binding.
The 5' carbon is a sidegroup off the ring.
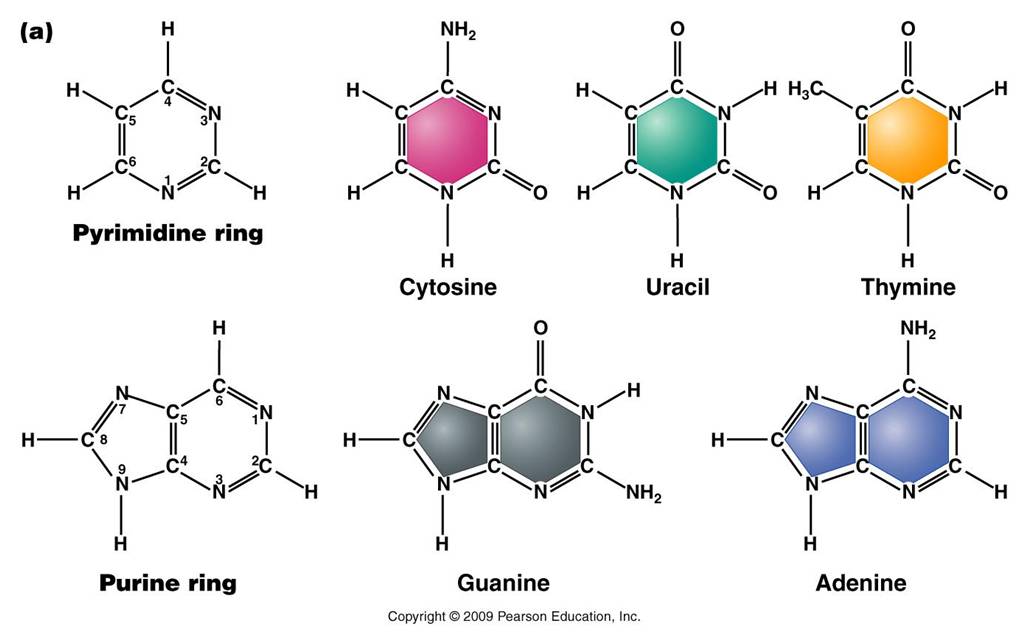
-
Nitrogenous Base: each nucleotide has a single nitrogenous
base attached to the 1' carbon of the sugar. This nitrogenous base may be
a double-ringed structure (purine) or a single ringed (pyrimidine) structure.
The purines are adenine (A) and guanine (G). The pyrimidines are thymine (T),
cytosine (C), and uracil (U). DNA nucleotides may carry A, G, C, or T. RNA
nucleotides carry either A, G, C, or U.
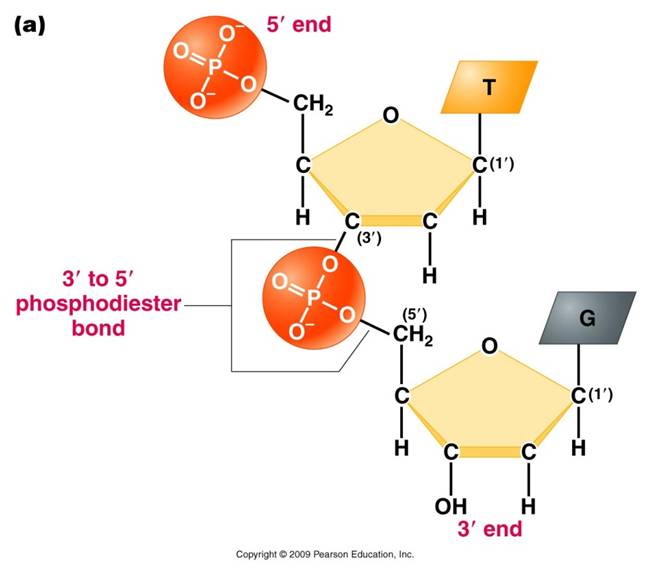 -
The third component of a nucleotide is a phosphate group,
which is attached to the 5' carbon of the sugar. When a nucleotide is incorporated
into a chain, it has a single phosphate group. However, nucleotides can occur
that have two or three phosphate groups (dinucleotides and trinucleotides).
ADP and ATP are important examples of these types of molecules. In fact, the
precursors of incorporated nucleotides are trinucleotides. When two phosphates
are cleaved, energy is released that can be used to add the remaining monophosphate
nucleotide to the nucleic acid chain.
-
The third component of a nucleotide is a phosphate group,
which is attached to the 5' carbon of the sugar. When a nucleotide is incorporated
into a chain, it has a single phosphate group. However, nucleotides can occur
that have two or three phosphate groups (dinucleotides and trinucleotides).
ADP and ATP are important examples of these types of molecules. In fact, the
precursors of incorporated nucleotides are trinucleotides. When two phosphates
are cleaved, energy is released that can be used to add the remaining monophosphate
nucleotide to the nucleic acid chain.
2. Polymerization
is by 'dehydration synthesis'
As with all other classes of biologically
important polymers, monomers are linked into polymers by dehydration synthesis.
In nucleic acid formation, this involves binding the phosphate group of one
nucleotide to the -OH group on the 3' carbon of the existing chain. For the
purposes of seeing how this reaction works, we can envision an H+ on one of
the negatively charged oxygens of the phosphate group. Then, a molceule of water
can be removed from these two -OH groups, leaving an oxygen binding the sugar
of one nucleotide to the phosphate of the next.
This creates a 'dinucleotide'. It
has a polarity/directionality; it is different at its ends. At one end, the
reactive group is the phosophate on the 5' carbon. This is called the 5' end
of the chain. At the other end, the reactive group is the free -OH on the 3'
carbon; this is the 3' end of the chain. So, a nucleic acid strand has a 5'
- 3' polarity.
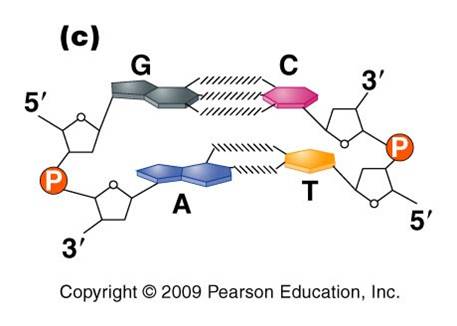 3.
Most DNA exists as a 'double helix' (ds-DNA) containing two linear nucleic acid
chains.
3.
Most DNA exists as a 'double helix' (ds-DNA) containing two linear nucleic acid
chains.
a. the
nitrogenous bases on the two strands are 'complementary' to each other,
and form weak hydrogen bonds between them. A always pairs with T, and C always
pairs with G. As such, there is always a double-ringed purine pairing with
a single-ringed pyrimidine, and the width of the double-helix is constant
over its entire length.
b. the
two strands (helices) are anti-parallel: they are arranged with opposite polarity. One strands points 5' - 3', while
the other points 3' - 5'. The direction of the pentose sugars and the type
of reactive group at the ends of the chains show this relationship.
4. RNA performs
a wide variety of functions in living cells:
a. m-RNA
(for "messenger") is the copy of
a gene. It is the
sequence of nitrogenous bases in m-RNA that is actually read by the ribosome
to determine the structure of a protein.
b. r-RNA
(for "ribosomal") is made the same way, as a copy of DNA. However, it is not carrying the recipe
for a protein; rather, it is functional as RNA. It is placed IN the Ribosome,
and it helps to ‘read’ the m-RNA.
c. t-RNA
(for "transfer") is also made as a copy of DNA, but it is also functional as an RNA molecule.
Its function is to bind to a specific amino acid and incorporate it into the
amino acid sequence as instructed by the m-RNA and ribosome.
B. Protein Synthesis
As we've already
mentioned, protein synthesis is fundamental to nearly everything a cell does.
Protein channels are used to transport large molecules across the membrane.
Almost all chemical reactions occuring in cells are catalyzed by protenaceous
enzymes, including those involved in energy harvest, DNA replication, and cell
division. Proteins perform important structural functions within cells and multicellular
organisms, too; such as the histone proteins in chromosomes, the proteins in
ribosomes, the collagen and elastin fibers that hold skin cells together, the
collagen on which calcium and phosphate is deposited in bone, the protein myofibrils
of actin and myosin in muscle cells, the neurotransmitters used for cell-cell
communication between neurons, and the enzymes that digest food in the stomach
and intestine of animals. So, proteins are fundamental to what cells and organisms
ARE, structurally, and what they DO functionally. As you know, the genetic information
determines the types of proteins a cell can make. The subset of proteins a cell
actually DOES make, and the timing of WHEN they are made, is determined by what
genes are "on" and what genes are "off" at a given time.
This regulation of gene activity is ALSO co-ordinated by proteins - called transcription
factors - that bind to DNA and promote or inhibit gene activity. So, proteins
also regulate protein synthesis. Hopefully you see just how important proteins
are to cells and organisms. So, the process of making these proteins is important,
too. It is no surprize, then, that a lot of the energy harvested by a cell is used to make proteins. The phosphate bonds in ATP are broken, and the energy that is released is used to form new bonds between amino acids. The chain of amino acids that is produced becomes a functional protein.
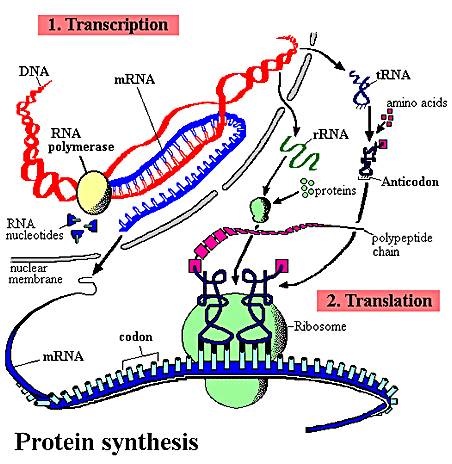 1.
Overview
1.
Overview
The sequence of nitrogenous
bases in a region of DNA is 'read' by a complex of enzymes that build a complementary
strand of RNA. This process of reading DNA and making RNA is called 'transcription'.
This is a great word for the process, as the message written in the language
of nucleic acids is copied in essentially the same language - the language of
nucleic acids. This RNA may be a recipe for a protein (m-RNA), or it may be
an RNA that will act on its own as t-RNA, mi-RNA, si-RNA, or be complexed with
proteins in the ribosome (r-RNA). Obviously, in "protein synthesis",
only the m-RNA is read to make a protein. However, the other molecules all play
a role. The sequence of nitrogenous bases in the m-RNA is then 'read' by a ribosome,
which links a specific sequence of amino acids together into a protein based
on that sequence of nitrogenous bases in the m-RNA. This process is called 'translation'.
This is a great choice of a word, too. Here the sequence of information written
in the language of nucleic acids is rewritten in a new language (hence, translation)
- the language of amino acids.
Many of the initial RNA products
have specific regions (introns) cut out of their sequence before they become
functional. This step is known as "RNA processing" or "RNA splicing".
Introns are present in nearly all eukaryotic RNA's, and are also in the DNA
genes that encode them. Up until a few years ago, the only introns in prokaryotes
had been found in t-RNA molecules of archaeans. More recently, however, introns
have been found in m-RNA and r-RNA molecules of a few eubacteria and a few more
archaeans. So, although they are rare in prokaryotes, we will describe a generic,
simplified process of protein synthesis that includes introns and RNA processing.
In addition to splicing the RNA product
of transcription, the initial protein product of translation may also be spliced
and modified before it becomes functional. In eukaryotes, this protein processing
often occurs in the Golgi apparatus.
The description presented here is
a simple model of protein synthesis. You will learn more complex aspects of
this process in Genetics.
2. The Process of Protein Synthesis
1. Transcription:
The DNA double helix is composed
of two anti-parallel complementary strands of DNA. There are sites recognized as "landing sites" by specific enzymes that are going to read the DNA and make RNA. These enzymes are RNA polymerases. They land, and make an RNA molecule complementary to one of the DNA strands (the one going in the 3-5 direction). There are sequences in the DNA that tell the enzyme where to stop, too, so the whole gene always gets read. This process makes all types of RNAs (m-RNA, r-RNA, t-RNA).
2. RNA splicing:
In this process,
so of the parts of the RNA get cut out (introns) and the meaningful message gets spliced together (exons).
3. Translation:
In this process,
amino acids are linked together into a protein by the ribosome. The particular sequence of amino
acids that are linked together is determined by the sequence of nitrogenous
bases in m-RNA. Scientists figured out the 'genetic code' in the 1960's, so we now know what 3-base combination of nucleotides in RNA codes for which amino acid. There are strting and stopping cues for this process, too; in fact the amino acid 'methionine' is always the first aminno acid put down, and the 3-base 'word' (codon) in the RNA that signals this start of translation is called the START codon. There are some 3-base codons that have no amino acid- -so when they are encountered by the ribosome, translation stops and the protein has ben made.
4. Protein Processing:
The initial protein product usually
needs to be modified to become functional. These modifications are termed "post-translational
modifications". First of all, the methionine is usually cut off - this
relieves an important constraint on the structure of functional proteins...
functional proteins DON'T all start with methionine! Then, the protein may be
spliced, or it may be bound with a sugar group (glycoprotein), lipid (lipoprotein),
nucleic acid (nucleoprotein), or another protein (quaternary protein). In eukaryotes,
much of this processing occurs in the Golgi apparatus.
3. Regulation
of Protein Synthesis
Aside from somatic mutations, all
the cells in a multicellular organism are genetically identical. So, the cells
in your retina, bone, muscle, and stomach lining all contain the same genes.
These cells perform different functions because they are reading different genes
and making different proteins. Your muscle has the gene for rhodopsin (a photoreceptive
pigment produced in the retina), but that gene is not transcribed in muscle
cells. In contrast, retinal cells have the genes for the muscle proteins actin
and myosin, but these genes are not transcribed. So, cell specialization and
the developmental process by which cells specialize from the fertilized egg
occurs by regulating this process of protein synthesis. Regulation can occur
at each of the steps described above.
The
process of transcription is regulated in several ways. First, the RNA polymerase
can be blocked from the promoter. This can happen because the gene is bound
to histones in a nucleosome, or is in a region of condensed 'heterochromatin',
or because other proteins called 'transcription factors' have bound to the DNA
- either at the promoter or between it and the gene, blocking the polymerase's
route. However, the binding of other transcription factors can increase the
affinity of the RNA polymerase for the promoter - increasing the probability
of transcription. Again, these transcription factors are proteins encoded by
other genes, and affected by other cellular processes. In this way, the action
of a gene can be co-ordinated with the activity of other genes in a complex
and interdependent manner. In addition, environmental cues from outside the
cell can, through signal transduction, affect the activity of transcription
factors and turn genes on or off. So, an organism can respond genetically to
environmental cues.
One way that differential splicing
can affect protein production is by changing the location of stop codons. For
example, suppose a stop codon occurs at the beginning of an intron. Then, suppose
that the intron is spliced incorrectly, after the location of this stop codon.
Now, the resulting functional m-RNA has a stop codon where it didn't before;
and translation will be terminated prematurely and no functional protein will
be produced.
Initial protein products can be cleaved
in different ways to produce different proteins, too.
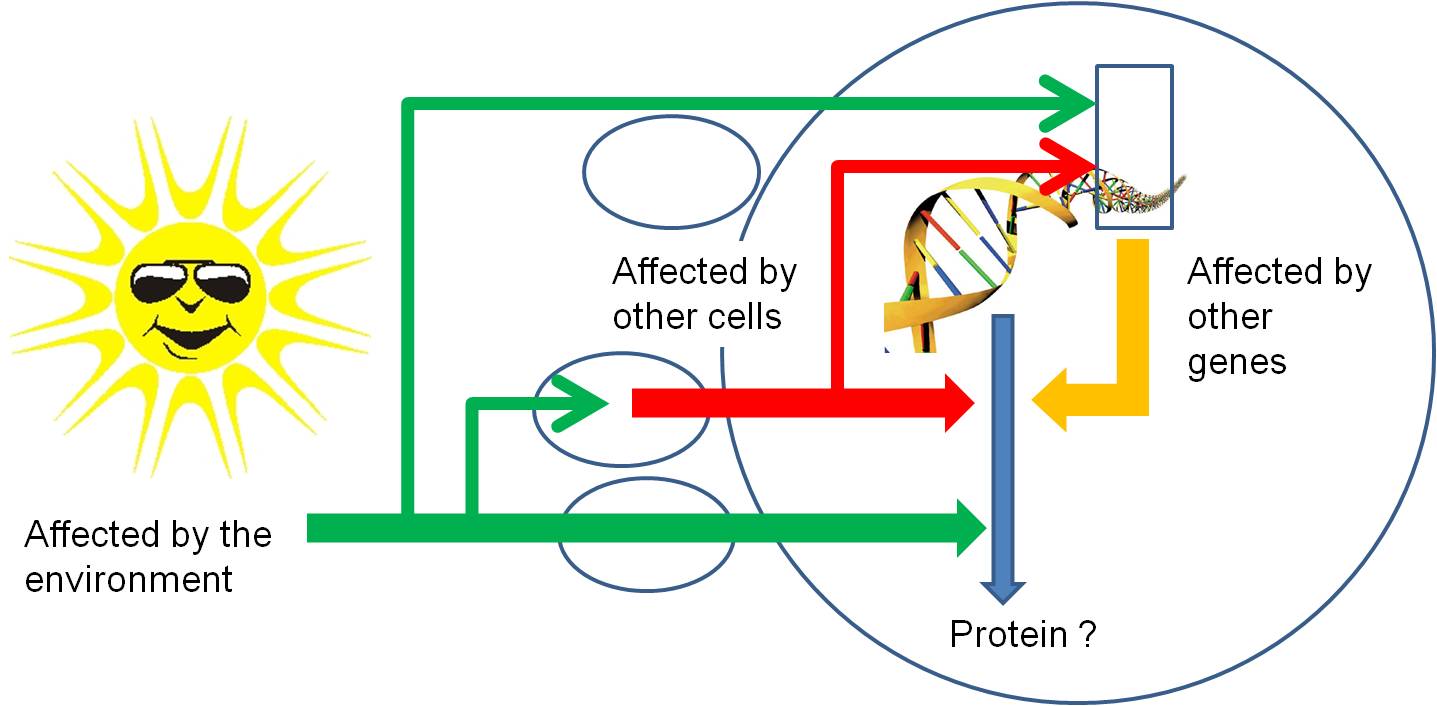
So, through all of these mechanisms,
protein synthesis can be stopped or stimulated, and the product can be modified.
Again, all of these regulatory pathways can be affected by environmental factors
or the proteins or mi/si-RNA's produced by other genes. So, gene activity is
affected by other things happening in the cell (turning other genes on and off)
, in other cells of the organism (through the production of hormones that act
as signal transducers), or environmental factors outside of the organism acting
directly on this gene, on other genes in this cell, or on other cells..
VII. Using Energy: Cell Reproduction
Cell division is the process of producing
two functional 'daughter' cells from one 'parental' cell. In order
for both of the daughter cells to have the full functional repertoire of the
original parental cell, they must be able to make the full complement of proteins
that the parent cell makes. In order for this to happen, they must both receive
the full complement of genetic information (DNA) in the parental cell. Hmmm....
how can they BOTH get the FULL COMPLEMENT of genetic information in the parental
cell? Well, in order for this to happen, the parental cell must duplicate its
DNA prior to cell division. This process of DNA replication produces two full
complements of genetic information. Then, this genetic information must be divided
evenly, in an organized manner, to insure that both daughter cells get the complete
complement of information (and not a duplication of some information or an omission
of other information). Cells that receive an incomplete complement of genetic
information will not be able to make all the proteins the parental cell made,
and may not be able to survive. So, again, DNA replication and the process of
mitosis are of great selective, adaptive value. Only cells that replicate and
divide their genetic information evenly, with only minor errors or inconsistencies,
will be likely to survive. These survivors will pass on the tendancy to replicate
and divide their genetic information evenly, as well. So, there is very strong
selection ( a very large selective advantage) for correct DNA replication and
equal chromosomal allocation during mitosis.
These processes of DNA replication
and mitosis are only two stages in the life of a cell. To place them in context,
it's useful to consider the full life of a cell, from it's production by the
division of its parental cell through to its own division.
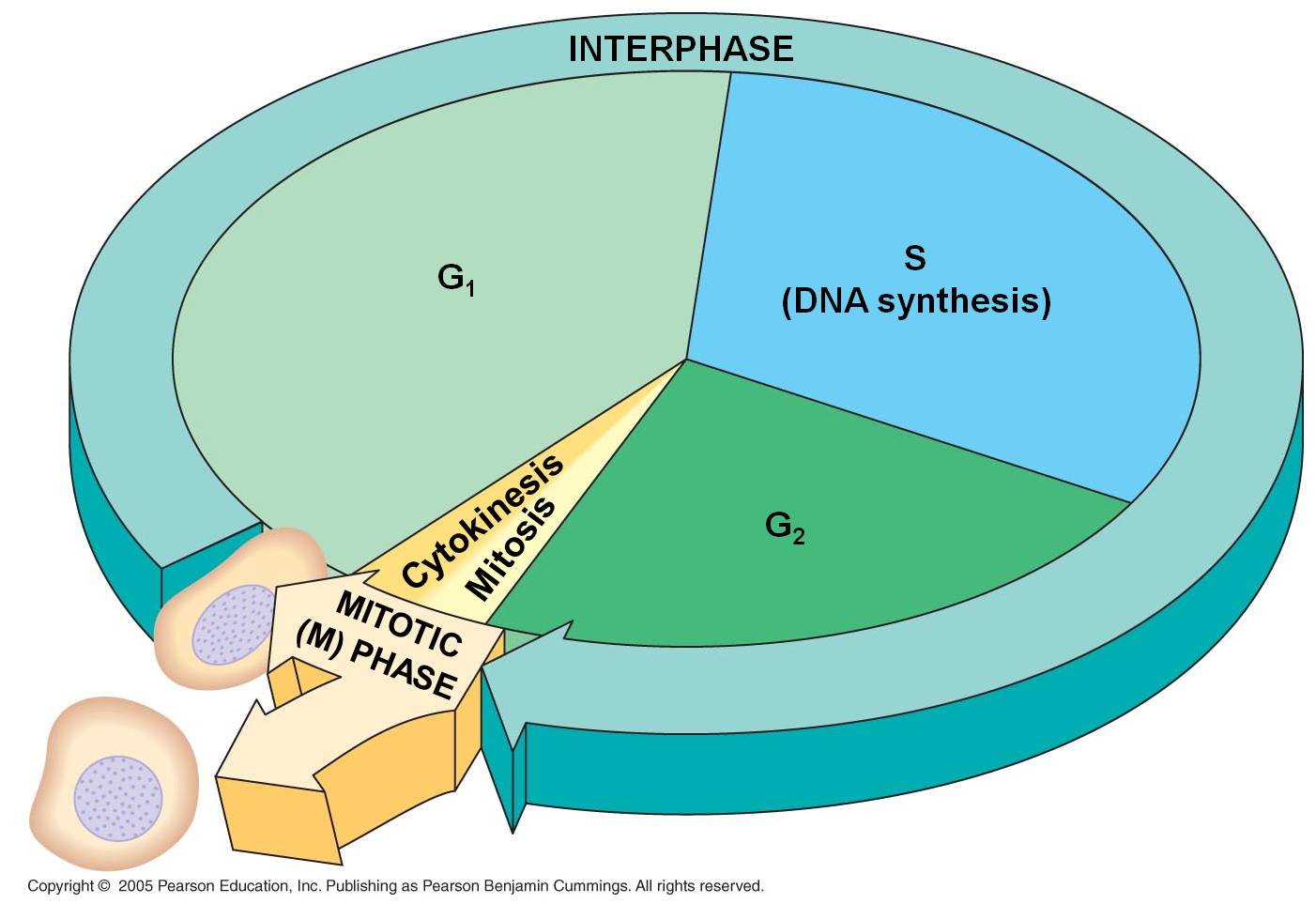 Mitosis and the Cell Cycle
Mitosis and the Cell Cycle
1. Interphase
- the 'interval' between divisions
a. G1
Our cell's life begins. That's sort
of a funny way to put it, because it seems to suggest that it is something new;
yet all of its constituents were part of the original parental cell. It is more
truly "1/2 an old cell with a full complement of DNA". Nevertheless,
it is an independent entity. In most protists, binary fission of the mitochondria
and chloroplasts occurs concurrently with the division of the nucleus during
mitosis, so the daughter cells have 'new' organelles, too. But in most multicellular
organisms, the allocation of organelles is largely a random process based on
how they are distributed in the cytoplasm during division. Then, the organelles
divide and 'repopulate' each daughter cell in G1.
The cell is roughly 1/2 the size
of the original parental cell. To grow to its appropriate size, it must synthesize
new biological molecules - and that means making the enzymes that will catalyze
those reactions. So, the DNA unwinds to the 'beads on a string' level, and the
genes between histones are available for transcription. When the DNA is unwound
('diffuse'), separate chromosomes cannot be seen with a light microscope. Rather,
the nucleus stains a uniform color except for one or several dark regions called
'nucleoli' (singular = nucleolus). These are areas were large amounts of r-RNA
are being synthesized and complexed with ribosomal proteins into functional
ribosomes. The ribosomes are exported from the nucleus to the cytoplasm, where
they will anchor to endoplasmic reticulum or the cytoskeleton.
Indeed, the G1 phase of a cell's
life is the most metabolically active period of it's life. It is growing in
size, and producing the proteins appropriate for its tissue type. Most cells
in multicellular organisms specialize during this period. Cells with very specific
structural adaptations to their specialized tissue type - like neurons with
long axons and muscle cells crammed with linear microfilaments - often remain
stalled in this stage after they become specialized; they do not divide again.
In this case, this stalled 'permanent' G1 phase is referred to a G0 ("G-nought').
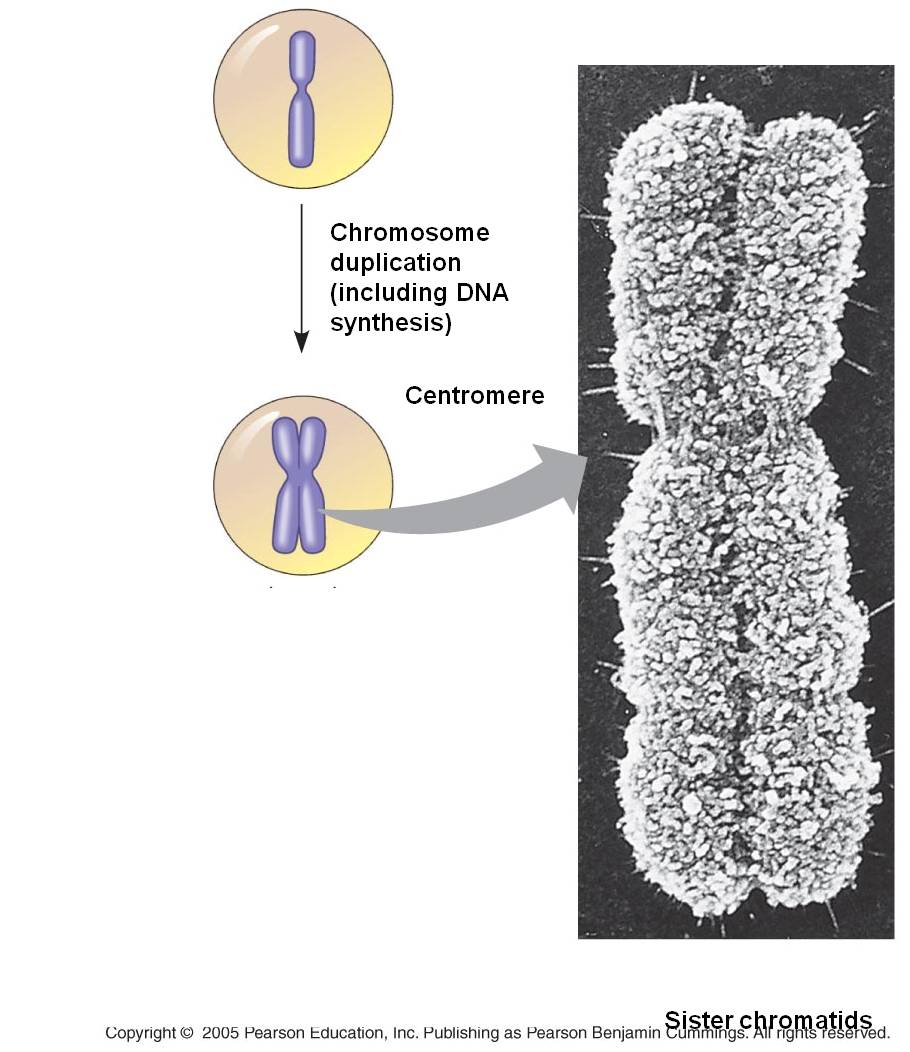 b.
S
b.
S
The S phase of the cell cycle is
when DNA replication occurs. The chromosomes are diffuse during this stage,
as well, so the enzymes (DNA polymerases) that replicate the DNA can access
the helices. Each double helix is separated, and the single strands are used
as templates for the formation of new helices on each template - changing one
double helix into two. Terminology becomes a bit ambiguous here. A DNA double
helix is equivalent to a "chromatid". A chromosome may have one chromatid
(in its unreplicated form) or two chromatids (in its replicated form). DNA replication
is a rather complicated process described in more detail below. The transition
from the G1 to the S phase is a very critical stage in a cell's life cycle,
signalling the cell's progression towards division. In eukaryotes it is called
a 'restriction point'. Once the S phase begins, the cell will proceed through
to mitosis. This transition is orchestrated by a complex interplay of transcription
factors that regulate the activity of "cell division cycle genes".
These genes produce cyclin proteins that vary in concentration through the cell
cycle. They bind with 'cyclin-dependent kinases' and these cdk-cyclin complexes
activate transcription factors that initiate the next phase of the cell cycle.
c. G2
After DNA replication is complete
the cell goes through another rapid period of growth in preparation for mitosis.
The DNA is checked again for damage caused and errors made during DNA replication.
Once again, p53 inhibits the transition to the mitotic phase, providing time
for this repair to take place. In cancer cells with mutations in p53, the G2
phase may be nearly eliminated, with the cell proceeding directly from DNA replication
to mitosis. CDK's bind to new cyclins, and these complexes active a different
set of proteins that initiate mitosis.
2. Mitosis
The process of mitosis can be summarized
as follows: the chromosomes condense, making it easier to divy them up evenly.
The replicated chromsomes are aligned in the middle of the cell by cytoskeletal
fibers. Each chromosome consistes of two identical double helices, called chromatids.
During the process of mitosis, these chromatids separate from each other, and
one double-helix from each chromosome is pulled to each end of the cell. The
membrane and cytoplasm are divided and the nuclear membrane reforms around the
chromosomes in each daughter cell. We will look at this process in more detail,
below.
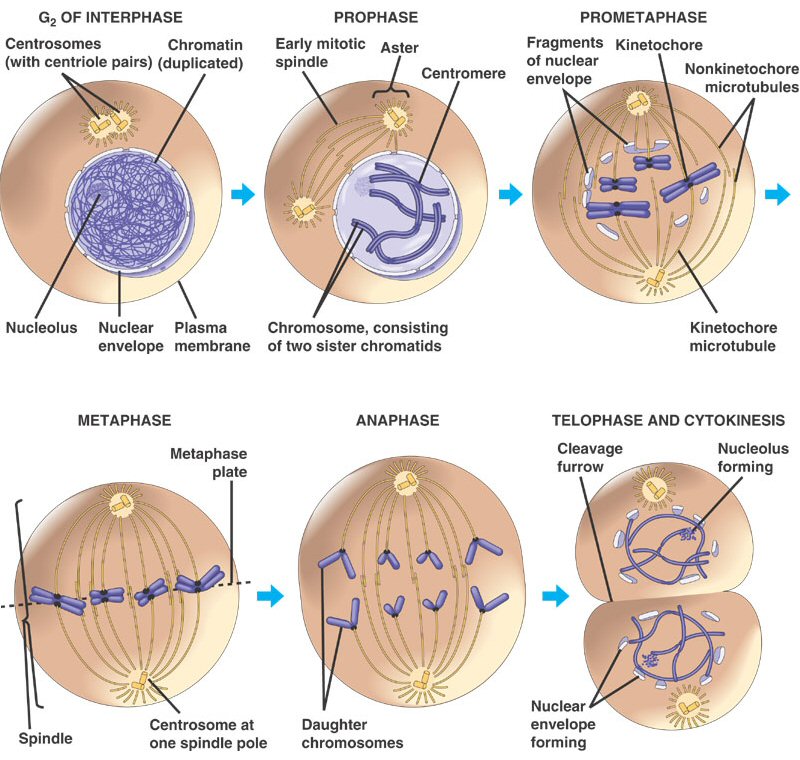
Mitosis is a continuous process of
chromosome condensation, chromatid separation, and cytoplasmic division. This
process is punctuated by particular events that are used to demarcate specific
stages. This process was first described by Walther Flemming in 1878, we he
developed new dyes and saw 'colored bodies' (chromo-somes) condensing and changing
position in dividing cells. He also coined the term 'mitosis' - the greek word
for thread - in honor of these thread-like structures.
1. Prophase: The transition from G2 to Prophase of Mitosis is marked by the condensation
of chromosomes.
2. Prometaphase: The chromosomes continue to condense, and the nuclear membrane disassembles.
The microfibers of the spindle apparatus attach to the kinetochores on the replicated
chromsomes.
3. Metaphase: The spindle aranges the chromosomes in the middle of the cell.
4. Anaphase: The proteins gluing sister chromatids together are metabolized, and the sister
chromatids are pulled by their spindle fibers to opposite poles of the cell.
It is important to appreciate that these separated chromatids (now individual,
unreplicated chromosomes) are idnetical to one another and identical to the
orignial parental chromosome (aside from unrepaired mutations).
5. Telophase: The cell continues to elongate, with a concentrated set of chromosomes at each
end. Nuclear membranes reform around each set of chromosomes, and the chromosomes
begin to decondense.
6. Cytokinesis: Cytokinesis is sometimes
considered a part of telophase. In this stage, the cytoplasm divides. In animal
cells, the membrane constricts along the cell's equator, causing a depression
or cleavage around the mid-line of the cell. This cleavage deepens until the
cells are pinched apart. In plants, vesicles from the golgi coalesce in the
middle of the cell, expanding to form a partition that divides the cell and
acts as a template for the deposition of lignin and cellulose that will form
the new cell wall between the cells.
As a consequence of this process,
two cells are produced from one parental cell, each having a complete complement
of genetic information - a copy of each original chromosome that was present
in the parental cell. Each of these cells now begins the G1 phase of interphase.
Study Questions:
1. Why is the lipid bilayer a barrier to water soluble molecules?
2.
Describe diffusion, facilitated diffusion, and active transport.
3. Show how the carbons in a glucose (C6) are separated in the steps of respiration.
4. Why is ATP made? Think of the money analogy.
5. What happens
in the electron transport chain?
6. How is oxygen
involved in the process of aerobic respiration?
7. Draw the Light
Indepedent reaction and describe the events that occur.
8. When, where,
and why is oxygen produced by photosynthesis? What is the primary function
of photosynthesis?
9. Draw
a DNA double helix as two lines, but showing three base pairs with A, T, C, G's.
10. What 'cues' determine where
transcription will start and stop?
11. Describe
translation: what is read, what is produced, and how is the 'genetic code' involved?.
12. Draw a
chromsome before and after replication; use the terms chromosome and chromatid.
13. Draw a
cell, 2n = 6, and show each of the stages of mitosis. Write a brief description
of the events of each stage.
 1.
Phospholipid Bilayer:
1.
Phospholipid Bilayer: 

 b.
Faciliated Diffusion:
Large polar molecules cannot diffuse across the lipid bilayer. However, they
can cross the membrane 'passively' from high to low concentration, through
integrated proteins channels. Some of these channels are rather unspecialized
'tubes', while other channels are rather specific and will only permit the
transport of certain classes of molecules. In any case, this is the way that
large polar molecules cross the membrane in response to their concentration
gradient (high to low).
b.
Faciliated Diffusion:
Large polar molecules cannot diffuse across the lipid bilayer. However, they
can cross the membrane 'passively' from high to low concentration, through
integrated proteins channels. Some of these channels are rather unspecialized
'tubes', while other channels are rather specific and will only permit the
transport of certain classes of molecules. In any case, this is the way that
large polar molecules cross the membrane in response to their concentration
gradient (high to low).  we will examine the energy harvesting
reactions that ALL living cells perform: Cellular Respiration. In other words, "what happens to the food you eat?" And, "what happens to the oxygen you breathe in?" All
living cells - eubacteria, archaea, protists, fungi, plants, and animals - can
harvest the energy contained in the chemical bonds of complex organic molecules. By breaking the covalent bonds between carbon atoms in these molecules, energy
is released. The energy released by these reactions must be trapped in other bonds or used to do work; otherwise it is lost as heat. So, cells perform coupled reactions to TRAP the energy. One reaction breaks down bonds in food, and the energy released is TRAPPED in new bods formed between
ADP and P --> making ATP. As such, some of the energy
in the covalent bonds of the initial organic molecules is transformed into chemical
energy in bonds of ATP. Carbon-carbon bonds are very strong and stable; enzymes can't break these bonds. These bonds are like a $100 bill--you can't use it everywhere...some stores won't take them. The energy must be converted to a 'lower denominationa; form' that can be used by all enzymes ('stores') in the cell. So, when the 5 carbon-carbon bonds in a glucose are broken, 36-38 bonds are made between ADP and P. And so, each of these bonds in ATP is MUCH weaker (contains less energy)... and most of the energy released by the breaking of the carbon-carbon bonds is lost as heat! Energy in this form is now available to all of the enzymes
in the cell, for catalyzing their own reactions (chemical energy) or doing work
like muscular contraction (mechanical energy) or pumping ions across a membrane
against their concentration gradient (active transport).
we will examine the energy harvesting
reactions that ALL living cells perform: Cellular Respiration. In other words, "what happens to the food you eat?" And, "what happens to the oxygen you breathe in?" All
living cells - eubacteria, archaea, protists, fungi, plants, and animals - can
harvest the energy contained in the chemical bonds of complex organic molecules. By breaking the covalent bonds between carbon atoms in these molecules, energy
is released. The energy released by these reactions must be trapped in other bonds or used to do work; otherwise it is lost as heat. So, cells perform coupled reactions to TRAP the energy. One reaction breaks down bonds in food, and the energy released is TRAPPED in new bods formed between
ADP and P --> making ATP. As such, some of the energy
in the covalent bonds of the initial organic molecules is transformed into chemical
energy in bonds of ATP. Carbon-carbon bonds are very strong and stable; enzymes can't break these bonds. These bonds are like a $100 bill--you can't use it everywhere...some stores won't take them. The energy must be converted to a 'lower denominationa; form' that can be used by all enzymes ('stores') in the cell. So, when the 5 carbon-carbon bonds in a glucose are broken, 36-38 bonds are made between ADP and P. And so, each of these bonds in ATP is MUCH weaker (contains less energy)... and most of the energy released by the breaking of the carbon-carbon bonds is lost as heat! Energy in this form is now available to all of the enzymes
in the cell, for catalyzing their own reactions (chemical energy) or doing work
like muscular contraction (mechanical energy) or pumping ions across a membrane
against their concentration gradient (active transport).  The
"splitting of glucose" (glyco-lysis) is probably an ancient metabolic
reaction; it is performed in the cytoplasm of ALL living cells from prokaryotes
to eukaryotes, and cells can perform this reaction in the presence OR absence
of oxygen gas. So, it seems likely that this was an important energy harvesting
reaction for ancient cells that lived before ~2 bya - before oxygen became abundant
in the oceans and atmosphere. As you can see in the flowchart, glycolysis is
not ONE reaction - it is a series of reactions catalyzed by a variety of enzymes.
The
"splitting of glucose" (glyco-lysis) is probably an ancient metabolic
reaction; it is performed in the cytoplasm of ALL living cells from prokaryotes
to eukaryotes, and cells can perform this reaction in the presence OR absence
of oxygen gas. So, it seems likely that this was an important energy harvesting
reaction for ancient cells that lived before ~2 bya - before oxygen became abundant
in the oceans and atmosphere. As you can see in the flowchart, glycolysis is
not ONE reaction - it is a series of reactions catalyzed by a variety of enzymes.
 Mitochondria have a double membrane
system like bacteria and chloroplasts, with an intermembrane space and matrix
within inner membrane.
Mitochondria have a double membrane
system like bacteria and chloroplasts, with an intermembrane space and matrix
within inner membrane.  Although all organisms (plants, animals, fungi, protists, bacteria) can harvest energy by breaking down organic molecules (Cellular Respiration), some have evolved a mechanism for transforming radiant energy in chemical bond energy. Photosynthesis
is that process of energy transformation. Again, although energy can neither be
created nor destroyed, it can be transformed. In the "Light Dependent Reaction"
radiant energy ('carried' by photons in light) is transformed into chemical
energy ('carried' by electrons). It requires an electron DONOR to provide electrons
that will 'carry' this energy. The energy 'carried' by this electron is used
to form a bond between ADP and P, creating ATP. Through this transfer, the electron
loses this energy. As we have discussed before, the phosphate bonds in ATP are
easily made and easily broken - that's why energy in this form of chemical bond
can be 'used' by all enzymes in the cell. However, ATP is readily hydrolyzed
in water...so it is difficult for a cell to build up a large amount of ATP before
it 'dissolves' to ADP and P again. To store large amounts of energy for a longer
time, the energy in ATP can be converted to a more stable molecule. In most
photosynthetic organisms, the catabolism of ATP is coupled to anabolic reactions
that bind carbon dioxide molecules together into stable molecules of glucose,
for longer term E storage. This also provides the cell with organic carbon that
it can use to make the other biologically important molecules. These are the
"Light Independent Reactions" of photosynthesis.
Although all organisms (plants, animals, fungi, protists, bacteria) can harvest energy by breaking down organic molecules (Cellular Respiration), some have evolved a mechanism for transforming radiant energy in chemical bond energy. Photosynthesis
is that process of energy transformation. Again, although energy can neither be
created nor destroyed, it can be transformed. In the "Light Dependent Reaction"
radiant energy ('carried' by photons in light) is transformed into chemical
energy ('carried' by electrons). It requires an electron DONOR to provide electrons
that will 'carry' this energy. The energy 'carried' by this electron is used
to form a bond between ADP and P, creating ATP. Through this transfer, the electron
loses this energy. As we have discussed before, the phosphate bonds in ATP are
easily made and easily broken - that's why energy in this form of chemical bond
can be 'used' by all enzymes in the cell. However, ATP is readily hydrolyzed
in water...so it is difficult for a cell to build up a large amount of ATP before
it 'dissolves' to ADP and P again. To store large amounts of energy for a longer
time, the energy in ATP can be converted to a more stable molecule. In most
photosynthetic organisms, the catabolism of ATP is coupled to anabolic reactions
that bind carbon dioxide molecules together into stable molecules of glucose,
for longer term E storage. This also provides the cell with organic carbon that
it can use to make the other biologically important molecules. These are the
"Light Independent Reactions" of photosynthesis. When
we think of photosynthesis, most of us think "plants". This is generally
correct, but very incomplete. First, there are some plants like Indian Pipe
(Monotropa uniflora) that do not photosynthesize. Although they evolved
from photosynthetic ancestors, they have adopted a parasitic lifestyle and no
longer harvest their own energy from sunlight. In addition, there are photosynthetic
protists (algae and Euglenozoans), and photosynthetic archaeans and eubacteria.
In fact, there are several animals that harbor photosynthetic symbionts, too.
Many corals (corals are animals) ingest algal cells and distribute them to their
tentacles. The algae photosynthesize, and excess sugars are passed to the coral
animal. These symbiotic algae give corals their spectacular colors. When stressed
by water polution or high water temperatures, the corals release their symbionts
and lose their color ("a phenomenon called "coral bleaching").
Long periods without their symbionts results in coral death.
When
we think of photosynthesis, most of us think "plants". This is generally
correct, but very incomplete. First, there are some plants like Indian Pipe
(Monotropa uniflora) that do not photosynthesize. Although they evolved
from photosynthetic ancestors, they have adopted a parasitic lifestyle and no
longer harvest their own energy from sunlight. In addition, there are photosynthetic
protists (algae and Euglenozoans), and photosynthetic archaeans and eubacteria.
In fact, there are several animals that harbor photosynthetic symbionts, too.
Many corals (corals are animals) ingest algal cells and distribute them to their
tentacles. The algae photosynthesize, and excess sugars are passed to the coral
animal. These symbiotic algae give corals their spectacular colors. When stressed
by water polution or high water temperatures, the corals release their symbionts
and lose their color ("a phenomenon called "coral bleaching").
Long periods without their symbionts results in coral death.  Photosynthesis
in prokaryotes occurs on the double-membrane system of these organisms. In eukaryotes,
photosynthesis occurs in organelles called chloroplasts. Chloroplasts have a
bacteria-like double membrane, and they have their own DNA. This DNA is more
similar in most respects to the DNA in free-living bacteria than to the DNA
in the nucleus of the eukaryotic cells they 'inhabit'. For these reasons, most
scientists accept the 'endosymbiotic theory' of chloroplast origins. This theory
states that chloroplasts in the cells of photosynthetic eukaryotes are descendants
of free-living photosynthetic bacteria. At some point in the early evolution
of protists, these photosynthetic bacteria were engulfed by not digested. Rather,
the host cells fed on the excess sugars produced by the internalized bacteria.
Eventually, as the result of gene exchange between the host and proto-chloroplasts,
the eukaryotic host and the prokaryotic symbiont became dependent on one another.
But chloroplasts can still live outside of cells for several days. Plants, evolving
from green algae ancestors, inherited these bacteria-like chloroplasts, too.
Photosynthesis
in prokaryotes occurs on the double-membrane system of these organisms. In eukaryotes,
photosynthesis occurs in organelles called chloroplasts. Chloroplasts have a
bacteria-like double membrane, and they have their own DNA. This DNA is more
similar in most respects to the DNA in free-living bacteria than to the DNA
in the nucleus of the eukaryotic cells they 'inhabit'. For these reasons, most
scientists accept the 'endosymbiotic theory' of chloroplast origins. This theory
states that chloroplasts in the cells of photosynthetic eukaryotes are descendants
of free-living photosynthetic bacteria. At some point in the early evolution
of protists, these photosynthetic bacteria were engulfed by not digested. Rather,
the host cells fed on the excess sugars produced by the internalized bacteria.
Eventually, as the result of gene exchange between the host and proto-chloroplasts,
the eukaryotic host and the prokaryotic symbiont became dependent on one another.
But chloroplasts can still live outside of cells for several days. Plants, evolving
from green algae ancestors, inherited these bacteria-like chloroplasts, too. Photosynthesis
is a critically important process in the evolution and diversity of life. Prior
to the evolution of photosynthesis, life was dependent on absorbing spontaneously
generated organic molecules, or preying on other cells. Neither of these sources
of energy was probably all that common and easy to find. Evolving the ability
to use sunlight as an energy source, which IS abundant and IS easy to find,
meant that life could grow, prosper, and radiate dramatically - almost anywhere
there was a light source. Indeed, it looks like photosynthesis evolved very
early in the history of life; the earliest fossils (stromatolites and filamentous
microfossils dating to ~3.5 by) look very similar to photosynthetic bacteria
that are alive today. When photosynthetic organisms became abundant, they provided
a food supply for a wider variety of heterotrophic cells. Heterotrophs could
then live anywhere phototrophs lived; they were not limited to those rare places
where biological molecules were forming spontaneously. So, complex bacterial
food webs evolved. These early photosynthetic organisms used a primitive form
of photosynthesis that did not produce oxygen as a waste product. So, even though
they flourished for a billion years, no oxygen was added to the atmosphere.
About 2.0 billion years ago, a 'modern' type of photosynthesis evolved that
used water as the electron donor and produced oxygen gas as a waste product.
The production of oxygen gas transformed the oceans (precipitating iron), and
eventually changed the atmosphere, as well. Although oxygen was probably a highly
toxic gas at first (because it is so reactive), life eventually evolved to tolerate
it and then to USE it in oxidative respiration. The evolution of aerobic respiration
allowed for more energy to be harvested from the catabolism of complex organic
molecules, and may have allowed for the evolution of more energy-demanding eukaryotes
and multicellular organisms. As you know, almost all food webs are ultimately
dependent on the photosynthetic organisms at the base of the "food chain"
(hydrothermal vent communities are a possible exception). We use this energy
to stick amino acids together to make our proteins, etc. Even the gas and oil
that powers our industrial societies was initally stored as glucose produced
by photosynthesis. Coal, gas, and oil are just fossilized plants - and we "burn"
that energy millions of years after it was converted from sunlight. We are powering
our societies with sunlight that hit the Earth millions of years ago. But not
only are you (and every other heterotroph) energetically dependant on photosynthetic
organisms for food, you are also indebted to them for changing the planet and
stimulating the evolution of eukaryotic and multicellular life. In short, there
are few processes more important to the history and current function of living
systems (and our petroleum-based economy) than photosynthesis.
Photosynthesis
is a critically important process in the evolution and diversity of life. Prior
to the evolution of photosynthesis, life was dependent on absorbing spontaneously
generated organic molecules, or preying on other cells. Neither of these sources
of energy was probably all that common and easy to find. Evolving the ability
to use sunlight as an energy source, which IS abundant and IS easy to find,
meant that life could grow, prosper, and radiate dramatically - almost anywhere
there was a light source. Indeed, it looks like photosynthesis evolved very
early in the history of life; the earliest fossils (stromatolites and filamentous
microfossils dating to ~3.5 by) look very similar to photosynthetic bacteria
that are alive today. When photosynthetic organisms became abundant, they provided
a food supply for a wider variety of heterotrophic cells. Heterotrophs could
then live anywhere phototrophs lived; they were not limited to those rare places
where biological molecules were forming spontaneously. So, complex bacterial
food webs evolved. These early photosynthetic organisms used a primitive form
of photosynthesis that did not produce oxygen as a waste product. So, even though
they flourished for a billion years, no oxygen was added to the atmosphere.
About 2.0 billion years ago, a 'modern' type of photosynthesis evolved that
used water as the electron donor and produced oxygen gas as a waste product.
The production of oxygen gas transformed the oceans (precipitating iron), and
eventually changed the atmosphere, as well. Although oxygen was probably a highly
toxic gas at first (because it is so reactive), life eventually evolved to tolerate
it and then to USE it in oxidative respiration. The evolution of aerobic respiration
allowed for more energy to be harvested from the catabolism of complex organic
molecules, and may have allowed for the evolution of more energy-demanding eukaryotes
and multicellular organisms. As you know, almost all food webs are ultimately
dependent on the photosynthetic organisms at the base of the "food chain"
(hydrothermal vent communities are a possible exception). We use this energy
to stick amino acids together to make our proteins, etc. Even the gas and oil
that powers our industrial societies was initally stored as glucose produced
by photosynthesis. Coal, gas, and oil are just fossilized plants - and we "burn"
that energy millions of years after it was converted from sunlight. We are powering
our societies with sunlight that hit the Earth millions of years ago. But not
only are you (and every other heterotroph) energetically dependant on photosynthetic
organisms for food, you are also indebted to them for changing the planet and
stimulating the evolution of eukaryotic and multicellular life. In short, there
are few processes more important to the history and current function of living
systems (and our petroleum-based economy) than photosynthesis. - Here's how it works: Light strikes the phosystems nested in the inner membrane
(called the 'thylakoid' membrane in chloroplasts). An electron in each photosystem
is excited and lost from the Mg in the chlorophyll molecule. The electrons are
accepted by particular electron acceptor molecules. The electron lost from PS
I is ultimately passed to NADP, which accepts a H+ to balance the charge, making
the high energy molecule, NADPH. The electron lost from PSII is passed to an
electron acceptor, and then to molecules in the electron transport chain. As
the electron is passed down the chain, ATP is produced by chemiosmosis (as described
above). When this electron has lost it's energy, it replaces the electron lost
from PS I. So, PS I is all set, and need not strip electrons from an electron
donor. However, PS II has lost an electron, and must replace this electron for
photosynthesis to continue. PSII strips electrons from H2O. Water
is split into oxygen, 2 H+, and 2 electrons. The electrons are passed to the
cholorophyll in PS II, excited by light, and energized. The oxygen reacts with
another oxygen atom to produce oxygen gas, which is released as a waste product.
The propose of photosynthesis is not "to produce oxygen". The purpose
of the light reaction of photosynthesis is to transform radiant energy into
chemcial energy, and produce ATP and NADPH. The two molecules, ATP and NADPH,
are the useful products. Again, oxygen gas is produced as a waste product when
electrons are stripped from water. The presence of oxygen in the oceans 2.5-2
billion years ago, indicated by the presence of sedimentary deposits with oxidized
iron (banded iron formations), indicates the evolution of this more advanced
type of photosynthesis that evolved in ancient photosynthetic bacteria.
- Here's how it works: Light strikes the phosystems nested in the inner membrane
(called the 'thylakoid' membrane in chloroplasts). An electron in each photosystem
is excited and lost from the Mg in the chlorophyll molecule. The electrons are
accepted by particular electron acceptor molecules. The electron lost from PS
I is ultimately passed to NADP, which accepts a H+ to balance the charge, making
the high energy molecule, NADPH. The electron lost from PSII is passed to an
electron acceptor, and then to molecules in the electron transport chain. As
the electron is passed down the chain, ATP is produced by chemiosmosis (as described
above). When this electron has lost it's energy, it replaces the electron lost
from PS I. So, PS I is all set, and need not strip electrons from an electron
donor. However, PS II has lost an electron, and must replace this electron for
photosynthesis to continue. PSII strips electrons from H2O. Water
is split into oxygen, 2 H+, and 2 electrons. The electrons are passed to the
cholorophyll in PS II, excited by light, and energized. The oxygen reacts with
another oxygen atom to produce oxygen gas, which is released as a waste product.
The propose of photosynthesis is not "to produce oxygen". The purpose
of the light reaction of photosynthesis is to transform radiant energy into
chemcial energy, and produce ATP and NADPH. The two molecules, ATP and NADPH,
are the useful products. Again, oxygen gas is produced as a waste product when
electrons are stripped from water. The presence of oxygen in the oceans 2.5-2
billion years ago, indicated by the presence of sedimentary deposits with oxidized
iron (banded iron formations), indicates the evolution of this more advanced
type of photosynthesis that evolved in ancient photosynthetic bacteria.
 The
primary reaction is called the Calvin-Benson
Cycle, and it works like this:
The
primary reaction is called the Calvin-Benson
Cycle, and it works like this:  Much of the energy harvested by a cell is used to make proteins. However, in order to understand protein synthesis, we must first describe the structure of DNA. DNA is the 'recipe' for proteins, so we need to take a little detour on our jounrey of how a cell works to describe the structure of DNA and chromosomes. DNA
is the genetic material in all forms of life (eubacteria, archaea, protists,
plants, fungi, and animals). Those quasi-living viruses vary in their genetic
material. Some have double-stranded DNA (ds-DNA) like living systems, while
others have ss-DNA, ss-RNA, and ds-RNA. RNA performs a wide array of functions
in living systems. Many of these functions have only been discovered in the
last few years.
Much of the energy harvested by a cell is used to make proteins. However, in order to understand protein synthesis, we must first describe the structure of DNA. DNA is the 'recipe' for proteins, so we need to take a little detour on our jounrey of how a cell works to describe the structure of DNA and chromosomes. DNA
is the genetic material in all forms of life (eubacteria, archaea, protists,
plants, fungi, and animals). Those quasi-living viruses vary in their genetic
material. Some have double-stranded DNA (ds-DNA) like living systems, while
others have ss-DNA, ss-RNA, and ds-RNA. RNA performs a wide array of functions
in living systems. Many of these functions have only been discovered in the
last few years. A.
DNA and RNA Structure
A.
DNA and RNA Structure -
Pentose (5 carbon) sugar: either ribose (RNA) or deoxyribose
(DNA). The carbons are numbered clockwise. The difference between the sugars
is that ribose has an -OH group on the 2' carbon, whereas deoxyriboes has
only 2 H groups and thus is "deoxygenated" relative to ribose. BOTH
sugars have an -OH group on the 3' carbon, which will be involved in binding.
The 5' carbon is a sidegroup off the ring.
-
Pentose (5 carbon) sugar: either ribose (RNA) or deoxyribose
(DNA). The carbons are numbered clockwise. The difference between the sugars
is that ribose has an -OH group on the 2' carbon, whereas deoxyriboes has
only 2 H groups and thus is "deoxygenated" relative to ribose. BOTH
sugars have an -OH group on the 3' carbon, which will be involved in binding.
The 5' carbon is a sidegroup off the ring. -
The third component of a nucleotide is a phosphate group,
which is attached to the 5' carbon of the sugar. When a nucleotide is incorporated
into a chain, it has a single phosphate group. However, nucleotides can occur
that have two or three phosphate groups (dinucleotides and trinucleotides).
ADP and ATP are important examples of these types of molecules. In fact, the
precursors of incorporated nucleotides are trinucleotides. When two phosphates
are cleaved, energy is released that can be used to add the remaining monophosphate
nucleotide to the nucleic acid chain.
-
The third component of a nucleotide is a phosphate group,
which is attached to the 5' carbon of the sugar. When a nucleotide is incorporated
into a chain, it has a single phosphate group. However, nucleotides can occur
that have two or three phosphate groups (dinucleotides and trinucleotides).
ADP and ATP are important examples of these types of molecules. In fact, the
precursors of incorporated nucleotides are trinucleotides. When two phosphates
are cleaved, energy is released that can be used to add the remaining monophosphate
nucleotide to the nucleic acid chain. 3.
Most DNA exists as a 'double helix' (ds-DNA) containing two linear nucleic acid
chains.
3.
Most DNA exists as a 'double helix' (ds-DNA) containing two linear nucleic acid
chains. 1.
Overview
1.
Overview
 Mitosis and the Cell Cycle
Mitosis and the Cell Cycle b.
S
b.
S