Cell Biology II: Harvesting Energy
I.
Cellular Respiration
Overview:
In this lecture, 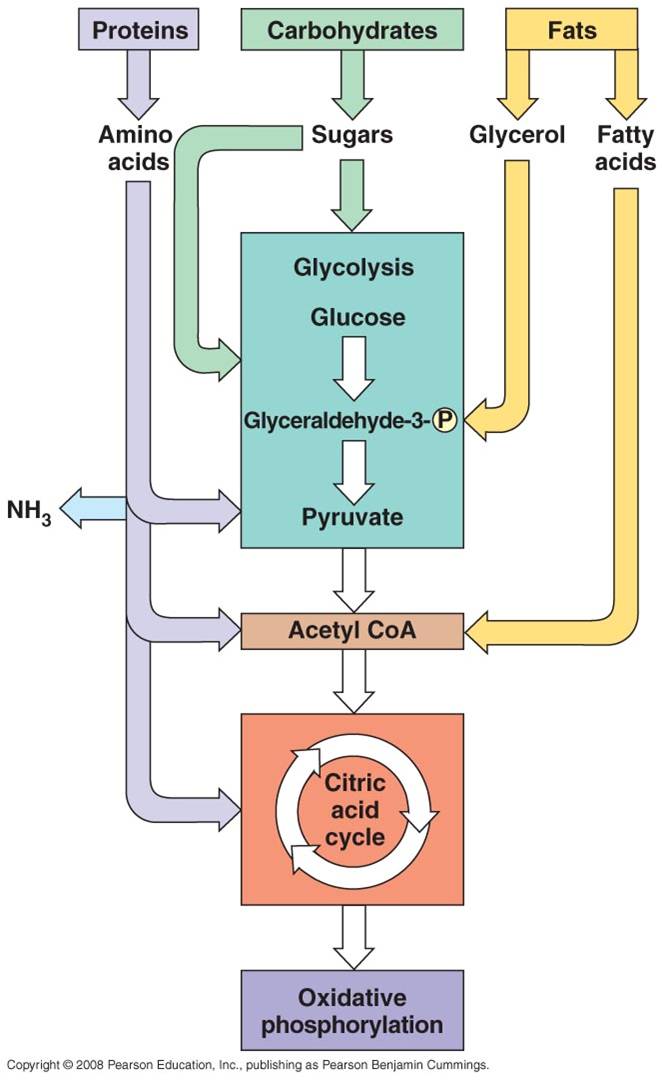 we will examine the energy harvesting
reactions that ALL living cells perform: Cellular Respiration. In other words, "what happens to the food you eat?" And, "what happens to the oxygen you breather in?" All
living cells - eubacteria, archaea, protists, fungi, plants, and animals - can
harvest the energy contained in the chemical bonds of complex organic molecules.
By breaking the covalent bonds between carbon atoms in these molecules, energy
is released. The energy released by these reactions must be trapped in other bonds or used to do work; otherwise it is lost as heat. So, cells perform coupled reactions to TRAP the energy. One reaction breaks down bonds in food, and the energy released is TRAPPED in new bods formed between
ADP and P --> making ATP. As such, some of the energy
in the covalent bonds of the initial organic molecules is transformed into chemical
energy in bonds of ATP. Carbon-carbon bonds are very strong and stable; enzymes can't break these bonds. These bonds are like a $100 bill--you can't use it everywhere...some stores won't take them. The energy must be converted to a 'lower denominationa; form' that can be used by all enzymes ('stores') in the cell. So, when the 5 carbon-carbon bonds in a glucose are broken, 36-38 bonds are made between ADP and P. And so, each of these bonds in ATP is MUCH weaker (contains less energy)... and most of the energy released by the breaking of the carbon-carbon bonds is lost as heat! Energy in this form is now available to all of the enzymes
in the cell, for catalyzing their own reactions (chemical energy) or doing work
like muscular contraction (mechanical energy) or pumping ions across a membrane
against their concentration gradient (active transport).
we will examine the energy harvesting
reactions that ALL living cells perform: Cellular Respiration. In other words, "what happens to the food you eat?" And, "what happens to the oxygen you breather in?" All
living cells - eubacteria, archaea, protists, fungi, plants, and animals - can
harvest the energy contained in the chemical bonds of complex organic molecules.
By breaking the covalent bonds between carbon atoms in these molecules, energy
is released. The energy released by these reactions must be trapped in other bonds or used to do work; otherwise it is lost as heat. So, cells perform coupled reactions to TRAP the energy. One reaction breaks down bonds in food, and the energy released is TRAPPED in new bods formed between
ADP and P --> making ATP. As such, some of the energy
in the covalent bonds of the initial organic molecules is transformed into chemical
energy in bonds of ATP. Carbon-carbon bonds are very strong and stable; enzymes can't break these bonds. These bonds are like a $100 bill--you can't use it everywhere...some stores won't take them. The energy must be converted to a 'lower denominationa; form' that can be used by all enzymes ('stores') in the cell. So, when the 5 carbon-carbon bonds in a glucose are broken, 36-38 bonds are made between ADP and P. And so, each of these bonds in ATP is MUCH weaker (contains less energy)... and most of the energy released by the breaking of the carbon-carbon bonds is lost as heat! Energy in this form is now available to all of the enzymes
in the cell, for catalyzing their own reactions (chemical energy) or doing work
like muscular contraction (mechanical energy) or pumping ions across a membrane
against their concentration gradient (active transport).
All four classes of biological molecules
(carbo's, fats, proteins, and nucleic acids) are broken down for energy harvest.
The process of carbohydrate metabolism, however, is the central process.
Fats, proteins, and nucleic acids are broken into their monomers, these are
modified, and then these products can be shunted into the carbohydrate digestion
process. So, although we will focus on carbohydrate metabolism - and glucose
metabolism in particular - you should appreciate that all other polymers can
be broken down for energy harvest. And respiration not only harvests energy
- respiration also provides the monomers needed by the cell to build its own
biomolecules. So, when you digest protein, energy is harvested and the separated
amino acids can be used by your cells to make your DNA-specified proteins. This
is why a balanced diet is important - digestion of varied complex organic molecules
provides the different monomers and other essential vitamins and minerals (often
used as cofactors in reactions) that your cells require.
The metabolism of glucose can accur
in the presence of absence of oxygen. The first step is glycolysis, in which
the six-carbon sugar is split into 2 C3 molecules of pyruvate. The breaking
of this bond releases a small amount of energy. In the absence of oxygen, fermentation
occurs. The primary function of this "anaerobic" respiration is to
recyclce some chemicals needed to keep glycolysis going. So, anaerobic respiration,
including glycolysis and fermentation, breaks only a couple bonds and produces
only a small amount of energy. In the presence of oxygen, the pyruvates can
be completely oxidized. The C3 molecules are completely broken down into 3 one-carbon
molecules of carbon dioxide. The complete breakdown of the the pyruvates releases
much more energy. This is probably why aerobic organisms have come to dominate
the planet - they harvest more energy from the food they consume, and can use
this energy to survive and reproduce more effectively.
1. Glycolysis
a. the process:
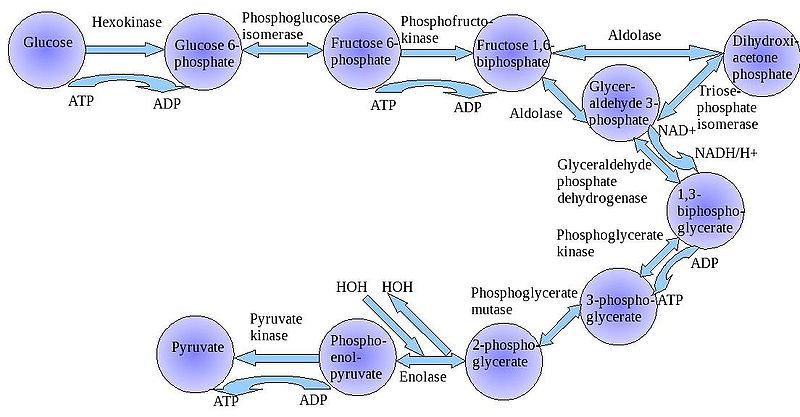 The
"splitting of glucose" (glyco-lysis) is probably an ancient metabolic
reaction; it is performed in the cytoplasm of ALL living cells from prokaryotes
to eukaryotes, and cells can perform this reaction in the presence OR absence
of oxygen gas. So, it seems likely that this was an important energy harvesting
reaction for ancient cells that lived before ~2 bya - before oxygen became abundant
in the oceans and atmosphere. As you can see in the flowchart, glycolysis is
not ONE reaction - it is a series of reactions catalyzed by a variety of enzymes.
For our purposes here, we will consider the primary "inputs" and "outputs"
of the entire reaction, rather than concerning ourselves with each step.
The
"splitting of glucose" (glyco-lysis) is probably an ancient metabolic
reaction; it is performed in the cytoplasm of ALL living cells from prokaryotes
to eukaryotes, and cells can perform this reaction in the presence OR absence
of oxygen gas. So, it seems likely that this was an important energy harvesting
reaction for ancient cells that lived before ~2 bya - before oxygen became abundant
in the oceans and atmosphere. As you can see in the flowchart, glycolysis is
not ONE reaction - it is a series of reactions catalyzed by a variety of enzymes.
For our purposes here, we will consider the primary "inputs" and "outputs"
of the entire reaction, rather than concerning ourselves with each step.
Through this series of reactions,
the six-carbon glucose is modified and split into 2 C3 molecules
of pyruvate. Glycolysis requires an input of energy to "get the reaction
going". This activation energy is provided by 2 ATP. A phosphate is transferred
from each ATP to the terminal carbons on the glucose. These phosphates destabilize
the glucose, and also give it a charge - it will not diffuse back across the
lipid bilayer. The splitting of the molecule releases energy and high energy
electrons. Some of the energy is used to phosphorylate 4 ADP--> 4 ATP. Thus,
although 2 ATP were used to start the reaction, there is a net gain of 2 ATP.
The high energy electrons are accepted by an important molecule called NAD (nicotinamide
adenine dinucleotide). With the acceptance of an electron, each NAD becomes
negative charged (NAD-) and reacts with a H+ ion in solution - making NADH.
So, NAD is a low energy form of the molecule, and NADH is a high energy form
of the molecule.
So, for our purposes here, we can
summarize glycolysis as:
glucose (C6) + 2 ATP +
2NAD ----> 2 pyruvate (C3) + 4 ATP + 2NADH
video
b. Requirements:
In order for glycolysis to occur
(and all subsequent glucose metabolism!!!) the cell must have all three
reactants - Glucose, ATP, and NAD. Obviously, if glucose is absent
then the cell starves. That's moot. And, As glycolysis proceeds, there
is always a net surplus of ATP produced by previous glycolysis reactions. But
what about NAD? As glycolysis proceeds, extra ATP is produced that can be used
in subsequent reactions to keep metabolism going. However, NAD is used up and
converted to NADH. If NAD is not present, glycolysis stops (very BAD).
c. Solutions:
So, NADH must give up its electrons
(and H+) to something else, so that the NAD can be recycled and used in glycolysis.
This happens two ways, depending on whether oxygen is present (aerobic respiration)
or absent (anearobic respiration). In the absence of oxygen, the NADH passed
the electron back to pyruvate, and either lactate or ethanol is formed. No new
energy is released from these reactions, so the energy produced by anaerobic
respiration is just what is produced in glycolysis - fermentation is just a
mechanism for the cell to recycle the NAD to keep glycolysis going. In
the presence of oxygen, the pyruvates can be metabolized more completely - splitting
all the carbon-carbon bonds and releaseing much more energy. Indeed, this is
the primary benefit of aerobic respiration; but the recycling of NAD also happens
during the process.
2. Aerobic Respiration
Anaerobic respiration and lactate
fermentation break only a single carbon-carbon bond in the glucose molecule.
As such, only a small amount of the energy present in a glucose molecule is
released. In aerobic respiration, the glucose is completely oxidized - all six
carbon atoms are broken apart, and much more energy is released (and harvested).
Oxygen is a very reactive gas - it
oxidizes things - stripping electrons from other molecules and breaking bonds.
Combustion is an oxidative process, and it can occur spontaneously, without
an ignition source. So, if combustible material heats up above its ignition
temperature, and if a strong oxidative agent like oxygen is present, it will
ignite. Gasoline is a long hydrocarbon polymer. When raised above it's ignition
point, it will combust. The gasoline will be oxidized to CO2 and
H2O - and the breaking of the carbon-carbon bonds that occurs during
this process will release energy. A gallon of gasoline contains ALOT of bonds
and ALOT of energy; and if it is released all at once, the energy is difficult
to control or use - you get an uncontrolled explosion. In a car's internal combustion
engine, very small amounts of gasoline are ignited in sequence, by spark plugs,
causing a little explosion in each cylinder that pushes the piston that turns
the crankshaft that turns the wheels of the car. In a diesel engine, there are
no spark plugs and no spark; the fuel ignites when the temperature exceeds its
ignition point when placed under high pressure when the piston rises. By controling
the reaction, by oxidizing just a little at a time, the energy released can
be used to do work.
All organic molecules - sugars, nucleic
acids, proteins, and fats - can be oxidized in the presence of a strong oxidative
agent like oxygen gas. In fact, after oxygenic photosynthesis evolved and after
iron precipitated out of suspension, when oxygen began to accumulate in the
oceans and atmosphere, it was probably toxic to life. As described above, most
of the most ancient forms of life - like archaeans - are obligate anaerobes
and are still poisoned by oxygen to this day. Some life forms evolved specific
enzymes and 'anti-oxidants' to protect themselves from the oxidative effects
of oxygen gas, and they tolerated this new environment. Some of their descendants
evolved a mechanism to use the oxidative effects of oxygen gas in a productive
way - in a way that released the energy in organic molecules in controlled reactions
where the energy could be harvested effectively and used to do chemical work.
These aerobic organisms came to dominate the planet, probably in part because
they could harvest more energy from the food they consumed; energy they could
use to grow, survive, and reproduce. Aerobic respiration probably evolved about
2.0 billion years ago, in response to the increase in oxygen concentrations.
Shortly thereafter, aerobically respiring bacteria were engulfed by other cells
but not consumed; rather, the host cells used the ATP produced by these energetically
efficient 'bacteria'. These host cells were the first eukaryotes, that evolved
about 900 million years ago. Their energetic endosymbionts evolved into the
mitochondria present in living eukaryotic cells. Like chloroplasts, mitochondria
have their own bacteria-like chromosome and a bacteria-like double membrane
system. In eukaryotes, the process of aerobic respiration occurs within these
organelles. From these ancestral eukaryotes, some absorbed photosynthetic endosymbionts,
too; these because the photosynthetic algae and their descendants, the plants.
SO - PLEASE KNOW THIS: all eukaryotes (except a couple wierd protists like Giardia),
HAVE MITOCHONDRIA - that includes protists, fungi, animals, and PLANTS. Photosynthetic
eukaryotoes, the agae and plants, ALSO HAVE chloroplasts.
a. Overall Process:
- Pyruvates are broken down
into carbon dioxide.
- Energy that is released from
the complete breadown of the C-C bonds is used to make bonds in ATP (38).
- When bonds are broken, electrons
are released. They have to be accepted by another molecule. They are initally
accepted by NAD and FAD, which then take their "high energy" forms of NADH and
FADH2. Ultimately, they transfer this energy to ATP, give up
their electrons and H+, and are recylced as NAD and FAD. (That's important,
remember? We need to recycle that NAD so glycolysis - the first step in
this whole process - can continue.)
- Ultimately, the electrons
are passed to Oxygen O--, which then binds two hydrogen ions to balance charge
(forming water).
- Aerobic respiration is a
more complete breakdown of glucose, so it yields more ATP than glycolysis, alone
- In eukaryotes, this occurs
in a three step process in the mitochondria of cells.
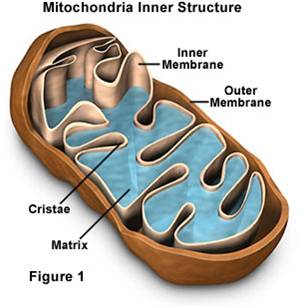 b.
Mitochondrial Structure:
b.
Mitochondrial Structure:
- Mitochondria have a double membrane
system like bacteria and chloroplasts, with an intermembrane space and matrix
within inner membrane.
- They have their own DNA, and they
replicate themselves by fission - they aren't 'made' by the cell.
- Given these observations, Lynn
Margulis hypothesized that these similarities were due to common ancestry, rather
than common environment. She raised this hypothesis as the endosymbiotic
hypothesis of eukaryote evolution, hypothesizing that eukaryotes acquired their
organelles by engulfing free-living bacteria and, rather than digesting them,
simply engulfed them and consumed their products (in this case the ATP that
the bacteria produce. The relationship is called symbiotic, because Margulis
hypothesized that the bacteria would also benefit by being in a stable environment
where the concentration of glucose was high (inside the cell).
- The most direct test of a hypothesis
of relatedness is DNA similarity. DNA only comes from parents, so similarities
imply a common source. When these tests were performed in the 1970's, her hypothesis
was confirmed. Additional tests with choloplasts and basal bodies (other
organielles in eukaryotes) also showed strong patterns of relatedness with free-living
bacteria. As such, we now refer to this tested model as the Endosymbiotic
Theory. We will describe this theory in more detail later in the term...
c. The Details:
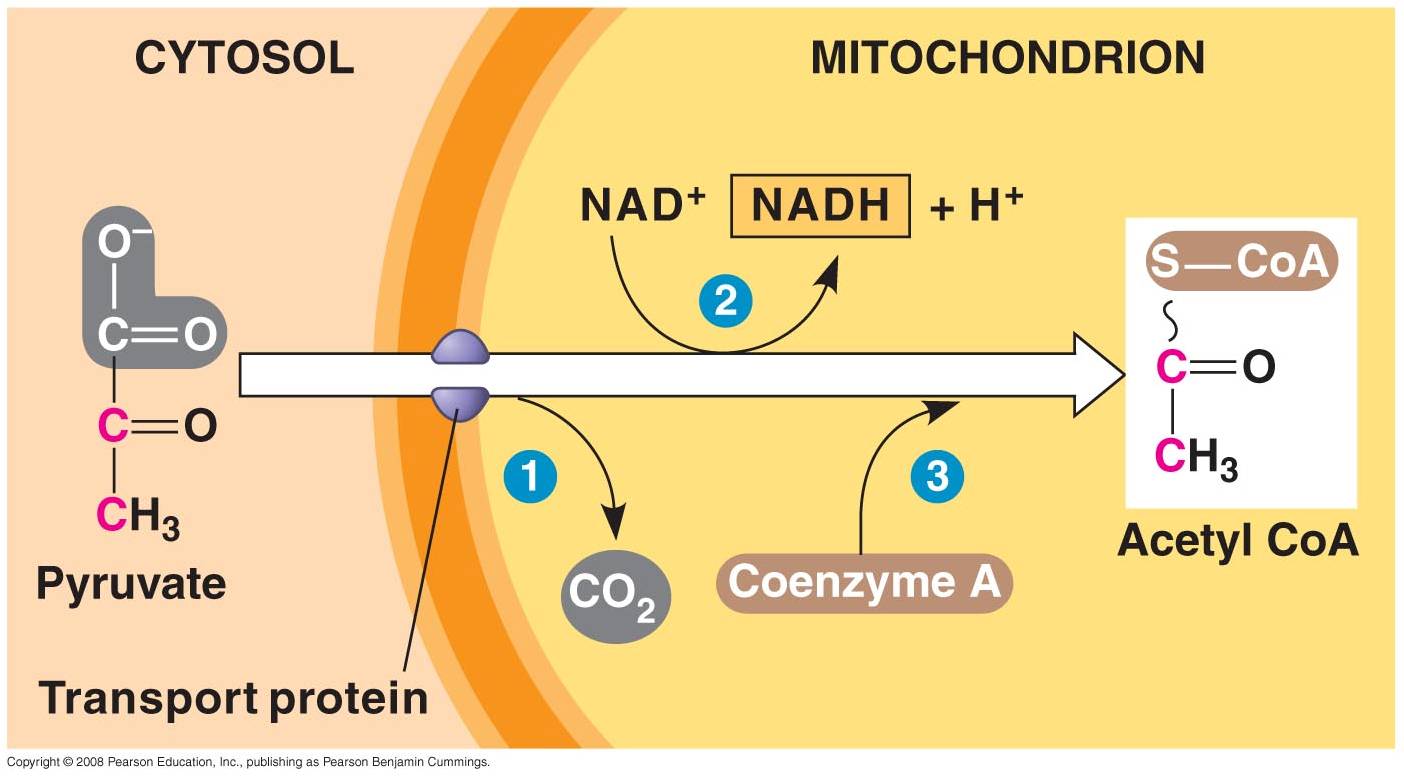 1. 'Gateway' Step:
1. 'Gateway' Step:
- Pyruvates cross both membranes
into the mitochondria and enter the 'matrix' - the cytoplasm of the organelle.
- Each pyruvate reacts with
a Coenzyme A molecule, and is split into a C2-CoA molecule and CO2.
(One C broken off).
- The electrons and energy
released are accepted by NAD, forming 1 NADH for each pyruvate used.
2. Krebs Cycle: 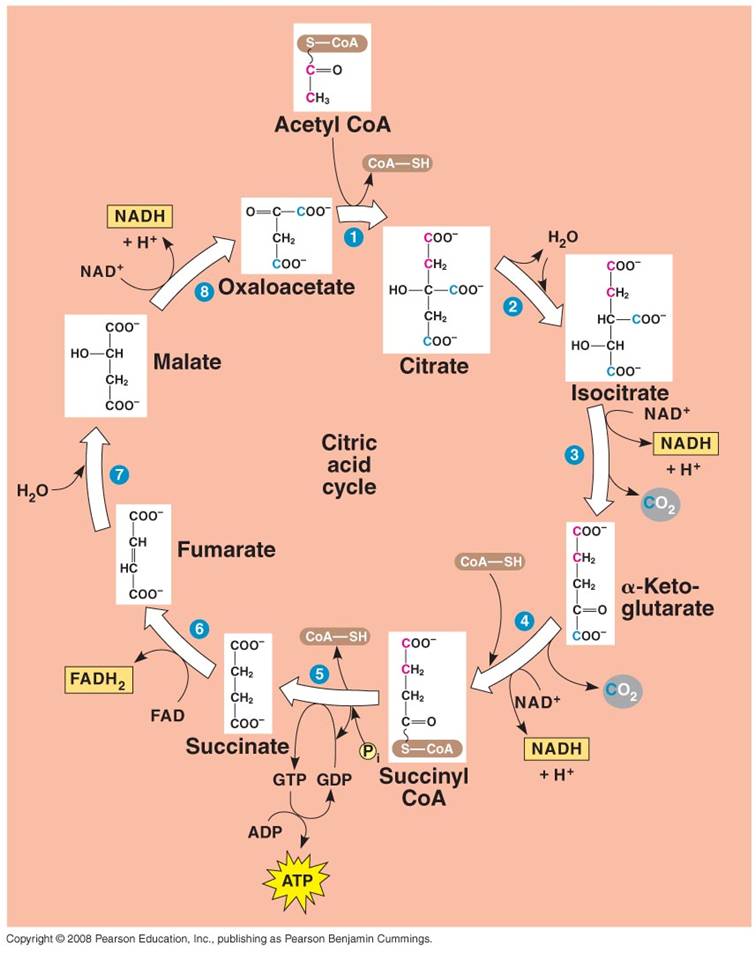
- Each C2-CoA reacts
with a C4 molecule (oxaloacetate).
- The C2 acetate
is transferred to the C4 molecule, forming a C6 molecule
of citrate. (CoA is released and recycled).
- Through a series of reactions,
the 2 'extra' C's are broken off as CO2 molecules and the C4
molecule is regenerated (Cycle).
- Some of the energy released
by the breaking of the C-bonds is used to make 1 ATP, 3 NADH, and 1 FADH2.
3. Electron Tranport Chain:
- Proteins nested in the inner
membrane of the mitochondria accept the electrons from NADH and FADH2
- The electrons are passed
from molecule to molecule, and some of the energy released is used to pump H+
ions across the inner membrane from the matrix to the intermembrane compartment
- Asteep concentration gradient
of H+ ions is formed... this represents chemical potential energy.
- When the ions flow through
protein channels associated with ATP-synthesizing enzymes in the membrane, this
potential energy is transformed into chemical energy in bonds between ADP and
P, making ATP.
- When the electrons reach
a low energy state, they are accepted by oxygen in the matrix and H+ ions react
with the O-- to form water. This is the ONLY use of oxygen in the process
- as an electron acceptor.
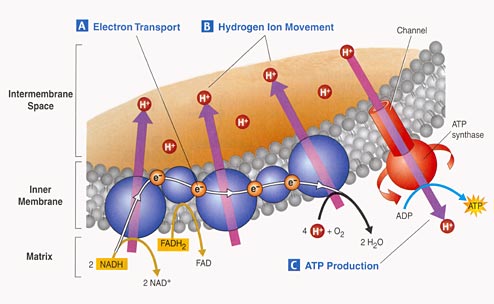
- 34 to 36 ATP are made from the energy tranferred from NADH and FADH2
molecules produced in the Krebs Cycle.
4. Summary of Glucose Metabolism
Glycolysis - net yield is:
Glycolysis: 2 ATP (net), NADH
Gateway:
2 NADH
Krebs: 2 ATP,
6 NADH, 2 FADH2
ETC:
34 ATP
Total: 38 ATP
5. Metabolizing
other Biomolecules
All Biomolecules represent stores of
energy that can be harvested when the molecules are digested.
a.
Fats:
- Glycerol broken from fatty acids; glycerol (3C) fed into glycolysis and
are modified into pyruvates (C3). - the Fatty Acids are broken
down into C2 groups that are modified to react with CoA - they
are shuinted to the Krebs Cycle - these reacts are reversible, so if there
is a surplus of C2-CoA, it can react to form fatty acids ---> energy
consumed in carbo's can be stored as bonds in fat.
b.
Proteins:
- Broken into Amino Acids which, depending on their structure, can be shunted
into glycolysis, modified into pyruvate, or broken into acetate (C2).
- In all cases, the amine groups are cleaved, producing ammonia (NH3)
as a toxic waste. In mammals, this is converted into urea which must be diluted
in water for removal from the body (urine). Reptiles and birds convert it
to uric acid, which is expelled as a paste that does not require as much water
for dilution.
c.
Nucleic Acids: - ribose can be metabolized after coversion to
glucose.
II. Photosynthesis
Overview:
 Although all organisms can harvest energy by breaking down organic molecules (Cellular Respiration), some have evolved a mechanism for transforming radiant energy in chemical bond energy. Photosynthesis
is that process of energy transformation. Again, although energy can neither be
created nor destroyed, it can be transformed. In the "Light Dependent Reaction"
radiant energy ('carried' by photons in light) is transformed into chemical
energy ('carried' by electrons). It requires an electron DONOR to provide electrons
that will 'carry' this energy. The energy 'carried' by this electron is used
to form a bond between ADP and P, creating ATP. Through this transfer, the electron
loses this energy. As we have discussed before, the phosphate bonds in ATP are
easily made and easily broken - that's why energy in this form of chemical bond
can be 'used' by all enzymes in the cell. However, ATP is readily hydrolyzed
in water...so it is difficult for a cell to build up a large amount of ATP before
it 'dissolves' to ADP and P again. To store large amounts of energy for a longer
time, the energy in ATP can be converted to a more stable molecule. In most
photosynthetic organisms, the catabolism of ATP is coupled to anabolic reactions
that bind carbon dioxide molecules together into stable molecules of glucose,
for longer term E storage. This also provides the cell with organic carbon that
it can use to make the other biologically important molecules. These are the
"Light Independent Reactions" of photosynthesis.
Although all organisms can harvest energy by breaking down organic molecules (Cellular Respiration), some have evolved a mechanism for transforming radiant energy in chemical bond energy. Photosynthesis
is that process of energy transformation. Again, although energy can neither be
created nor destroyed, it can be transformed. In the "Light Dependent Reaction"
radiant energy ('carried' by photons in light) is transformed into chemical
energy ('carried' by electrons). It requires an electron DONOR to provide electrons
that will 'carry' this energy. The energy 'carried' by this electron is used
to form a bond between ADP and P, creating ATP. Through this transfer, the electron
loses this energy. As we have discussed before, the phosphate bonds in ATP are
easily made and easily broken - that's why energy in this form of chemical bond
can be 'used' by all enzymes in the cell. However, ATP is readily hydrolyzed
in water...so it is difficult for a cell to build up a large amount of ATP before
it 'dissolves' to ADP and P again. To store large amounts of energy for a longer
time, the energy in ATP can be converted to a more stable molecule. In most
photosynthetic organisms, the catabolism of ATP is coupled to anabolic reactions
that bind carbon dioxide molecules together into stable molecules of glucose,
for longer term E storage. This also provides the cell with organic carbon that
it can use to make the other biologically important molecules. These are the
"Light Independent Reactions" of photosynthesis.
 When
we think of photosynthesis, most of us think "plants". This is generally
correct, but very incomplete. First, there are some plants like Indian Pipe
(Monotropa uniflora) that do not photosynthesize. Although they evolved
from photosynthetic ancestors, they have adopted a parasitic lifestyle and no
longer harvest their own energy from sunlight. In addition, there are photosynthetic
protists (algae and Euglenozoans), and photosynthetic archaeans and eubacteria.
In fact, there are several animals that harbor photosynthetic symbionts, too.
Many corals (corals are animals) ingest algal cells and distribute them to their
tentacles. The algae photosynthesize, and excess sugars are passed to the coral
animal. These symbiotic algae give corals their spectacular colors. When stressed
by water polution or high water temperatures, the corals release their symbionts
and lose their color ("a phenomenon called "coral bleaching").
Long periods without their symbionts results in coral death.
When
we think of photosynthesis, most of us think "plants". This is generally
correct, but very incomplete. First, there are some plants like Indian Pipe
(Monotropa uniflora) that do not photosynthesize. Although they evolved
from photosynthetic ancestors, they have adopted a parasitic lifestyle and no
longer harvest their own energy from sunlight. In addition, there are photosynthetic
protists (algae and Euglenozoans), and photosynthetic archaeans and eubacteria.
In fact, there are several animals that harbor photosynthetic symbionts, too.
Many corals (corals are animals) ingest algal cells and distribute them to their
tentacles. The algae photosynthesize, and excess sugars are passed to the coral
animal. These symbiotic algae give corals their spectacular colors. When stressed
by water polution or high water temperatures, the corals release their symbionts
and lose their color ("a phenomenon called "coral bleaching").
Long periods without their symbionts results in coral death.
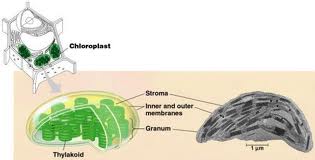 Photosynthesis
in prokaryotes occurs on the double-membrane system of these organisms. In eukaryotes,
photosynthesis occurs in organelles called chloroplasts. Chloroplasts have a
bacteria-like double membrane, and they have their own DNA. This DNA is more
similar in most respects to the DNA in free-living bacteria than to the DNA
in the nucleus of the eukaryotic cells they 'inhabit'. For these reasons, most
scientists accept the 'endosymbiotic theory' of chloroplast origins. This theory
states that chloroplasts in the cells of photosynthetic eukaryotes are descendants
of free-living photosynthetic bacteria. At some point in the early evolution
of protists, these photosynthetic bacteria were engulfed by not digested. Rather,
the host cells fed on the excess sugars produced by the internalized bacteria.
Eventually, as the result of gene exchange between the host and proto-chloroplasts,
the eukaryotic host and the prokaryotic symbiont became dependent on one another.
But chloroplasts can still live outside of cells for several days. Plants, evolving
from green algae ancestors, inherited these bacteria-like chloroplasts, too.
Photosynthesis
in prokaryotes occurs on the double-membrane system of these organisms. In eukaryotes,
photosynthesis occurs in organelles called chloroplasts. Chloroplasts have a
bacteria-like double membrane, and they have their own DNA. This DNA is more
similar in most respects to the DNA in free-living bacteria than to the DNA
in the nucleus of the eukaryotic cells they 'inhabit'. For these reasons, most
scientists accept the 'endosymbiotic theory' of chloroplast origins. This theory
states that chloroplasts in the cells of photosynthetic eukaryotes are descendants
of free-living photosynthetic bacteria. At some point in the early evolution
of protists, these photosynthetic bacteria were engulfed by not digested. Rather,
the host cells fed on the excess sugars produced by the internalized bacteria.
Eventually, as the result of gene exchange between the host and proto-chloroplasts,
the eukaryotic host and the prokaryotic symbiont became dependent on one another.
But chloroplasts can still live outside of cells for several days. Plants, evolving
from green algae ancestors, inherited these bacteria-like chloroplasts, too.
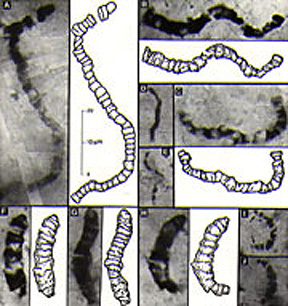 Photosynthesis
is a critically important process in the evolution and diversity of life. Prior
to the evolution of photosynthesis, life was dependent on absorbing spontaneously
generated organic molecules, or preying on other cells. Neither of these sources
of energy was probably all that common and easy to find. Evolving the ability
to use sunlight as an energy source, which IS abundant and IS easy to find,
meant that life could grow, prosper, and radiate dramatically - almost anywhere
there was a light source. Indeed, it looks like photosynthesis evolved very
early in the history of life; the earliest fossils (stromatolites and filamentous
microfossils dating to ~3.5 by) look very similar to photosynthetic bacteria
that are alive today. When photosynthetic organisms became abundant, they provided
a food supply for a wider variety of heterotrophic cells. Heterotrophs could
then live anywhere phototrophs lived; they were not limited to those rare places
where biological molecules were forming spontaneously. So, complex bacterial
food webs evolved. These early photosynthetic organisms used a primitive form
of photosynthesis that did not produce oxygen as a waste product. So, even though
they flourished for a billion years, no oxygen was added to the atmosphere.
About 2.0 billion years ago, a 'modern' type of photosynthesis evolved that
used water as the electron donor and produced oxygen gas as a waste product.
The production of oxygen gas transformed the oceans (precipitating iron), and
eventually changed the atmosphere, as well. Although oxygen was probably a highly
toxic gas at first (because it is so reactive), life eventually evolved to tolerate
it and then to USE it in oxidative respiration. The evolution of aerobic respiration
allowed for more energy to be harvested from the catabolism of complex organic
molecules, and may have allowed for the evolution of more energy-demanding eukaryotes
and multicellular organisms. As you know, almost all food webs are ultimately
dependent on the photosynthetic organisms at the base of the "food chain"
(hydrothermal vent communities are a possible exception). We use this energy
to stick amino acids together to make our proteins, etc. Even the gas and oil
that powers our industrial societies was initally stored as glucose produced
by photosynthesis. Coal, gas, and oil are just fossilized plants - and we "burn"
that energy millions of years after it was converted from sunlight. We are powering
our societies with sunlight that hit the Earth millions of years ago. But not
only are you (and every other heterotroph) energetically dependant on photosynthetic
organisms for food, you are also indebted to them for changing the planet and
stimulating the evolution of eukaryotic and multicellular life. In short, there
are few processes more important to the history and current function of living
systems (and our petroleum-based economy) than photosynthesis.
Photosynthesis
is a critically important process in the evolution and diversity of life. Prior
to the evolution of photosynthesis, life was dependent on absorbing spontaneously
generated organic molecules, or preying on other cells. Neither of these sources
of energy was probably all that common and easy to find. Evolving the ability
to use sunlight as an energy source, which IS abundant and IS easy to find,
meant that life could grow, prosper, and radiate dramatically - almost anywhere
there was a light source. Indeed, it looks like photosynthesis evolved very
early in the history of life; the earliest fossils (stromatolites and filamentous
microfossils dating to ~3.5 by) look very similar to photosynthetic bacteria
that are alive today. When photosynthetic organisms became abundant, they provided
a food supply for a wider variety of heterotrophic cells. Heterotrophs could
then live anywhere phototrophs lived; they were not limited to those rare places
where biological molecules were forming spontaneously. So, complex bacterial
food webs evolved. These early photosynthetic organisms used a primitive form
of photosynthesis that did not produce oxygen as a waste product. So, even though
they flourished for a billion years, no oxygen was added to the atmosphere.
About 2.0 billion years ago, a 'modern' type of photosynthesis evolved that
used water as the electron donor and produced oxygen gas as a waste product.
The production of oxygen gas transformed the oceans (precipitating iron), and
eventually changed the atmosphere, as well. Although oxygen was probably a highly
toxic gas at first (because it is so reactive), life eventually evolved to tolerate
it and then to USE it in oxidative respiration. The evolution of aerobic respiration
allowed for more energy to be harvested from the catabolism of complex organic
molecules, and may have allowed for the evolution of more energy-demanding eukaryotes
and multicellular organisms. As you know, almost all food webs are ultimately
dependent on the photosynthetic organisms at the base of the "food chain"
(hydrothermal vent communities are a possible exception). We use this energy
to stick amino acids together to make our proteins, etc. Even the gas and oil
that powers our industrial societies was initally stored as glucose produced
by photosynthesis. Coal, gas, and oil are just fossilized plants - and we "burn"
that energy millions of years after it was converted from sunlight. We are powering
our societies with sunlight that hit the Earth millions of years ago. But not
only are you (and every other heterotroph) energetically dependant on photosynthetic
organisms for food, you are also indebted to them for changing the planet and
stimulating the evolution of eukaryotic and multicellular life. In short, there
are few processes more important to the history and current function of living
systems (and our petroleum-based economy) than photosynthesis.
A. Step 1: The Light Dependent Reaction
AGAIN, the purpose of the light-dependent reaction is to convert radiant energy
to chemical energy. Obviously, light must be present; so this reaction "depends"
on sunlight. There is one
group of Archaeans that performs photosynthesis (Halobacteria), but their process
of harvesting light energy seems quite different from the process in eubacteria
and chloroplasts in eukarya and probably evolved independently. Within the eubacteria,
there are also a wide variety of photosynthetic processes. We will focus on
a couple major types and make reference to others as we go. 1. PRIMITIVE
SYSTEMS: 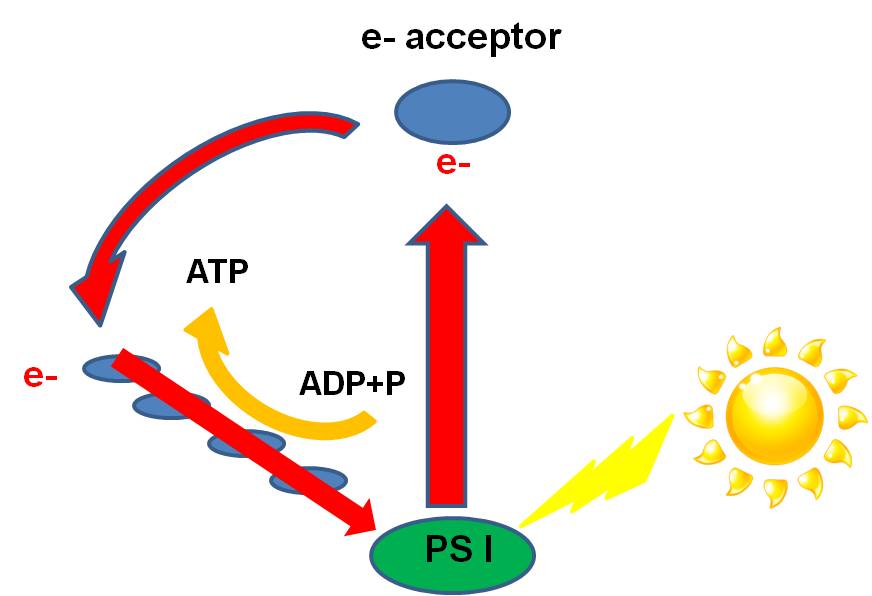 a.
cyclic phosphorylation in "purple non-sulphur" and "green non-sulpher"
bacteria:Like all
bacteria, they have a double membrane (two bilayers). Proteins nested within
the inner membrane form "reaction centers (also called "photosystems")
and "electron transport chains" (ETC's) used in photosynthesis. This
inner membrane is often highly convoluted, increasing the surface area and the
number of reaction centers and ETC's that can be imbedded. Each reaction
center contains proteins arrayed around molecules of bacteriochlorophyll, which
contain atoms of Magnesium. In the
presence of light, the photons transfer energy to these electrons. The electrons
are raised to a higher energy state, lost from
the atom, and transferred to an 'electron acceptor molecule' in the inner membrane
of the bacterium, which transfers the electron the the electron transport chain. When a high-energy electron
is transferred down the chain, protons
(H+) follow ('electrostatically') and are pumped across the inner membrane into
the intramembrane space. This build-up of H+ ions in the intermembrane space
creates an electrostatic charge differential across the membrane. There are
closed protein channels that, when opened, allow the H+ to flood through in
response to the charge gradient. This electric discharge energy is used by the
enzyme ATP-synthetase to add a phosphate group to ADP, making ATP. This is called
'chemiosmotic synthesis' or 'chemiosmosis'. So, what has happened is that the
passage of an electron -excited by light energy - has been used to 'pump protons'
into the intermembrane space, establishing an H+ ion charge gradient. The flow
of H+ ions through protein channels transforms this electric energy to chemical
bond energy in the form of a bond between ADP and P--> ATP. The
high-energy electron is then passed down the electron transport chain. and ATP
is produced. The electron, having lost its energy, can be recycled back to the
Mg atom. This cyclic production of ATP, powered by sunlight, is called cyclic
phosphorylation. As discussed below, these odd bacteria do not perform the light
independent pathways. In other words, they do not use the energy in ATP to make
glucose. This has two interesting consequences. First, it means they can't rely
on photosynthesis, alone, for energy harvest, because ATP isn't stable enough
to last over the course of an evening. So, they must also 'eat' - they are heterotrophs,
and can harvest energy from the food they ingest. The other consequence is discussed
below.
a.
cyclic phosphorylation in "purple non-sulphur" and "green non-sulpher"
bacteria:Like all
bacteria, they have a double membrane (two bilayers). Proteins nested within
the inner membrane form "reaction centers (also called "photosystems")
and "electron transport chains" (ETC's) used in photosynthesis. This
inner membrane is often highly convoluted, increasing the surface area and the
number of reaction centers and ETC's that can be imbedded. Each reaction
center contains proteins arrayed around molecules of bacteriochlorophyll, which
contain atoms of Magnesium. In the
presence of light, the photons transfer energy to these electrons. The electrons
are raised to a higher energy state, lost from
the atom, and transferred to an 'electron acceptor molecule' in the inner membrane
of the bacterium, which transfers the electron the the electron transport chain. When a high-energy electron
is transferred down the chain, protons
(H+) follow ('electrostatically') and are pumped across the inner membrane into
the intramembrane space. This build-up of H+ ions in the intermembrane space
creates an electrostatic charge differential across the membrane. There are
closed protein channels that, when opened, allow the H+ to flood through in
response to the charge gradient. This electric discharge energy is used by the
enzyme ATP-synthetase to add a phosphate group to ADP, making ATP. This is called
'chemiosmotic synthesis' or 'chemiosmosis'. So, what has happened is that the
passage of an electron -excited by light energy - has been used to 'pump protons'
into the intermembrane space, establishing an H+ ion charge gradient. The flow
of H+ ions through protein channels transforms this electric energy to chemical
bond energy in the form of a bond between ADP and P--> ATP. The
high-energy electron is then passed down the electron transport chain. and ATP
is produced. The electron, having lost its energy, can be recycled back to the
Mg atom. This cyclic production of ATP, powered by sunlight, is called cyclic
phosphorylation. As discussed below, these odd bacteria do not perform the light
independent pathways. In other words, they do not use the energy in ATP to make
glucose. This has two interesting consequences. First, it means they can't rely
on photosynthesis, alone, for energy harvest, because ATP isn't stable enough
to last over the course of an evening. So, they must also 'eat' - they are heterotrophs,
and can harvest energy from the food they ingest. The other consequence is discussed
below.
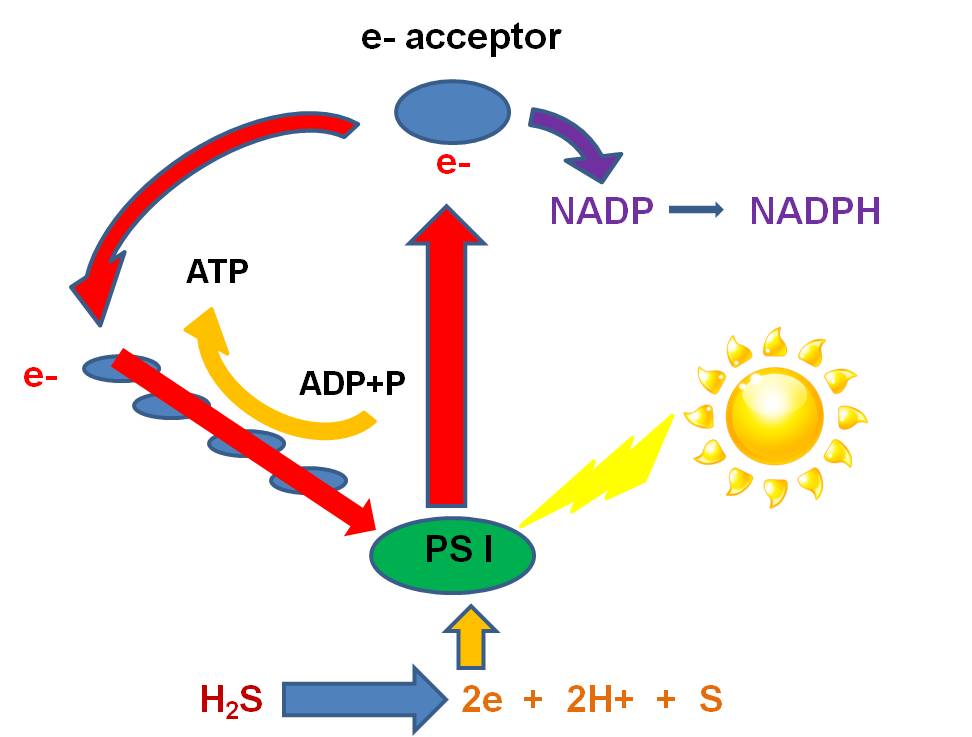 b.
"green sulphur" and "purple
sulphur" bacteria that
use sulphides as the electron donors:
b.
"green sulphur" and "purple
sulphur" bacteria that
use sulphides as the electron donors:
When the excited electron is recieved by the 'electron acceptor',something else
can happen. Instead of the Electron Acceptor giving the electron to the ETC,
it can give the electron to NADP... another 'energy transport molecule' like
ADP. When this happens, the NADP gains energy and a negative charge and is NADP-.
It reacts with free H+ ions that are always present in aqueous solutions (you
should know why...), to make the high energy transport molecule, NADPH. In this
case, the electron isn't returned to the Magnesium.... photosynthesis would
stop, unless the photosystem can strip electrons from other molecules in solution.
There are several groups of primitive
eubacteria ("green sulphur bacteria" and "purple sulphur bacteria")
that use sulfides (like hydrogen sulfide - H2S) as the electron donor.
Sulphur bacteria have photosystems
that strip electrons from Hydrogen Sulphide (H2S). This releases
2H+ ions and S as a waste product. So, sulphur bacteria that are still present
today photosynthesize in sulphur springs and do not produce oxygen as a waste
product. This explains an interesting geological pattern: The oldest fossil
life on record are photosynthetic bacteria that date to 3.8 billion years old.
However, the first evidence of oxygen in the Earth's atmosphere occurs at about
2 billion years ago. So, how can you now explain how there were photosynthetic
bacteria present for 1.8 billion years, without any oxygen being produced? Sulphur
bacteria. And they have another interesting characteristic - they are anaerobic
organisms poisoned by oxygen gas. So, not only don't they produce oxygen, but
they can only survive in its absence. All these factors suggest that they may
be similar to the first photosynthetic life forms that thrive in the anaerobic
environment of the early earth. There is a problem for them, however. These
bacteria can only survive in places where H2S is abundant - like
sulphur springs. These places are rare. If something evolved a system that could
strip electrons from a more abundant source, like water (H2O), then
these new organisms could exploit almost the whole planet - as 75% of the planet
is covered by H2O.
2. ADVANCED
SYSTEM: most
other photosynthetic bacteria (cyanobacteria), and photosynthetic eukaryotes.
- In photosynthetic Eukaryotes(photosynthetic protists and plants), these reactions
occur on the inner membrane of the Chloroplast - a specific membrane-bound organelle
very much like a bacterium within the larger eukaryotic cell. Indeed, as described
above, eukaryotic chloroplasts are probably the deescendants of free-living
cyanobacteria - with whom they share basic membrane structure and DNA similarity.
- In cyanobacteria and chloroplasts, there
are two types of reaction centers called "photosystems". The second
photosystem (PSII) has a lower electronegativity than the first, so it can exert
a 'stronger' pull and can strip electrons from WATER (which holds the electrons
more strongly than H2S does.) The splitting of water releases oxygen
gas as a waste product, so this type of photosynthesis is also called "oxygenic
photosynthesis".
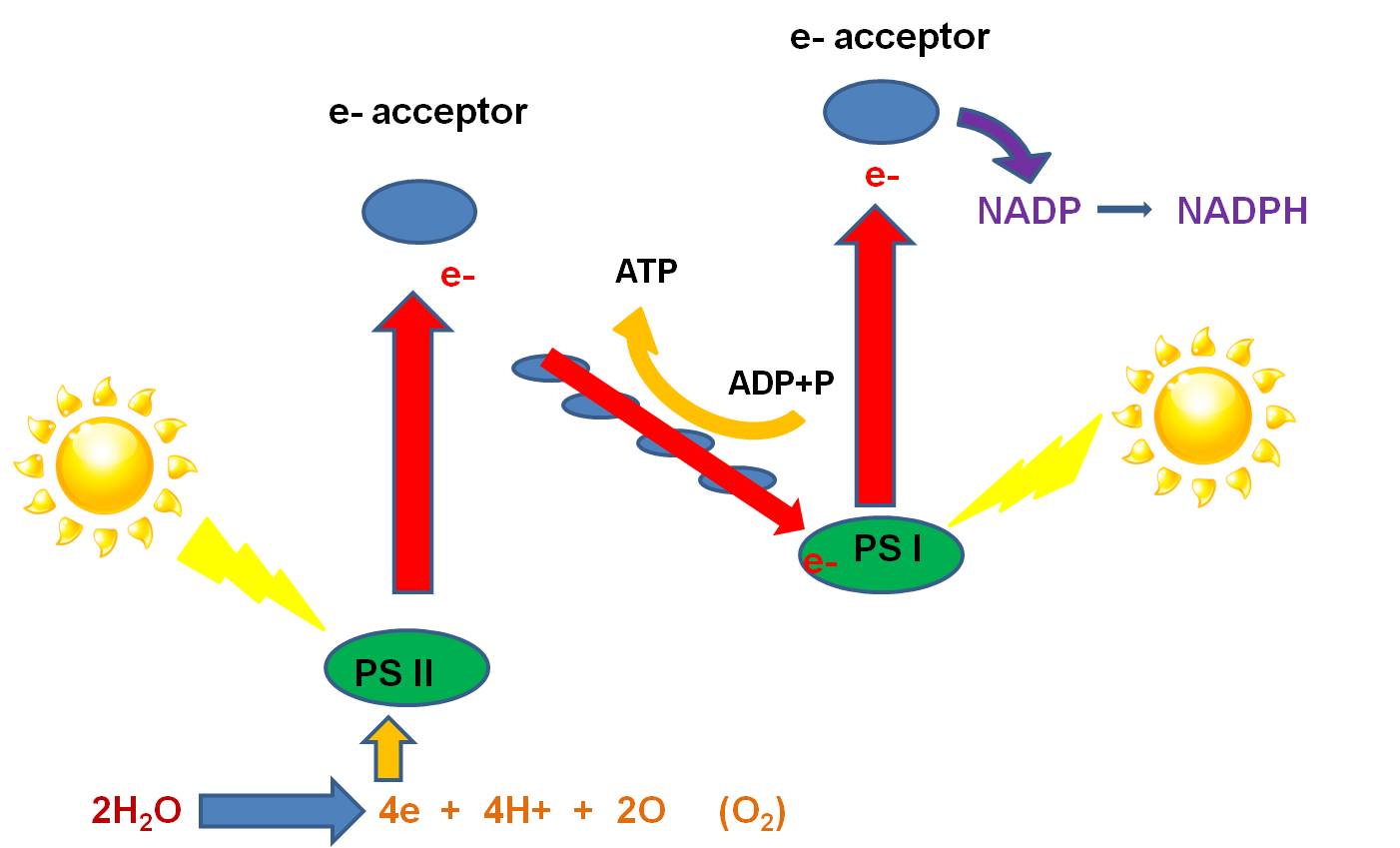 - Here's how it works: Light strikes the phosystems nested in the inner membrane
(called the 'thylakoid' membrane in chloroplasts). An electron in each photosystem
is excited and lost from the Mg in the chlorophyll molecule. The electrons are
accepted by partcular electron acceptor molecules. The electron lost from PS
I is ultimately passed to NADP, which accepts a H+ to balance the charge, making
the high energy molecule, NADPH. The electron lost from PSII is passed to an
electron acceptor, and then to molecules in the electron transport chain. As
the electron is passed down the chain, ATP is produced by chemiosmosis (as described
above). When this electron has lost it's energy, it replaces the electron lost
from PS I. So, PS I is all set, and need not strip electrons from an electron
donor. However, PS II has lost an electron, and must replace this electron for
photosynthesis to continue. PSII strips electrons from H2O. Water
is split into oxygen, 2 H+, and 2 electrons. The electrons are passed to the
cholorophyll in PS II, excited by light, and energized. The oxygen reacts with
another oxygen atom to produce oxygen gas, which is released as a waste product.
The propose of photosynthesis is not "to produce oxygen". The purpose
of the light reaction of photosynthesis is to transform radiant energy into
chemcial energy, and produce ATP and NADPH. The two molecules, ATP and NADPH,
are the useful products. Again, oxygen gas is produced as a waste product when
electrons are stripped from water. The presence of oxygen in the oceans 2.5-2
billion years ago, indicated by the presence of sedimentary deposits with oxidized
iron (banded iron formations), indicates the evolution of this more advanced
type of photosynthesis that evolved in ancient photosynthetic bacteria.
- Here's how it works: Light strikes the phosystems nested in the inner membrane
(called the 'thylakoid' membrane in chloroplasts). An electron in each photosystem
is excited and lost from the Mg in the chlorophyll molecule. The electrons are
accepted by partcular electron acceptor molecules. The electron lost from PS
I is ultimately passed to NADP, which accepts a H+ to balance the charge, making
the high energy molecule, NADPH. The electron lost from PSII is passed to an
electron acceptor, and then to molecules in the electron transport chain. As
the electron is passed down the chain, ATP is produced by chemiosmosis (as described
above). When this electron has lost it's energy, it replaces the electron lost
from PS I. So, PS I is all set, and need not strip electrons from an electron
donor. However, PS II has lost an electron, and must replace this electron for
photosynthesis to continue. PSII strips electrons from H2O. Water
is split into oxygen, 2 H+, and 2 electrons. The electrons are passed to the
cholorophyll in PS II, excited by light, and energized. The oxygen reacts with
another oxygen atom to produce oxygen gas, which is released as a waste product.
The propose of photosynthesis is not "to produce oxygen". The purpose
of the light reaction of photosynthesis is to transform radiant energy into
chemcial energy, and produce ATP and NADPH. The two molecules, ATP and NADPH,
are the useful products. Again, oxygen gas is produced as a waste product when
electrons are stripped from water. The presence of oxygen in the oceans 2.5-2
billion years ago, indicated by the presence of sedimentary deposits with oxidized
iron (banded iron formations), indicates the evolution of this more advanced
type of photosynthesis that evolved in ancient photosynthetic bacteria.
3. SUMMARY
OF LIGHT REACTIONS:
- Radiant energy excites electrons,
which are passed down an electron transport chain and ATP is made.
- The first organisms to evolve
this process probably used H2S as the donor of these electrons.
These sulphur bacteria do not produce oxygen as a waste product; they produce
sulphur.
- The evolution of PSII was very
important, because photosynthetic organisms could use H2O as the
electron donor and were no loner limited to living in places where H2S
was abundant. These organisms that use water as the electron donor produce
oxygen as a waste product, and the geologic record suggests that this process
evolved about 2.3-2.0 bya.
- Ultimately, the electrons are
passed to NADP, making NADPH.
- Water + ADP + NADP --> in the
presence of light --> ATP + NADPH + O2 (waste)
B. Step
2: The Light Independent Reactions:
The purpose of the "Light Independent
Reactions" is to convert the chemical energy in fragile ATP and NADPH molecules
into a more stable energy form by building covalent bonds between carbon atoms
to make glucose. In prokaryotes, these reactions occur in the cytoplasm of the
cell; in eukaryotes, these reactions occur in the stroma - or cytoplasm - of
the chloroplasts. It is important to appreciate that organisms using both primitive
and advanced light reactions perform the light independent reactions.
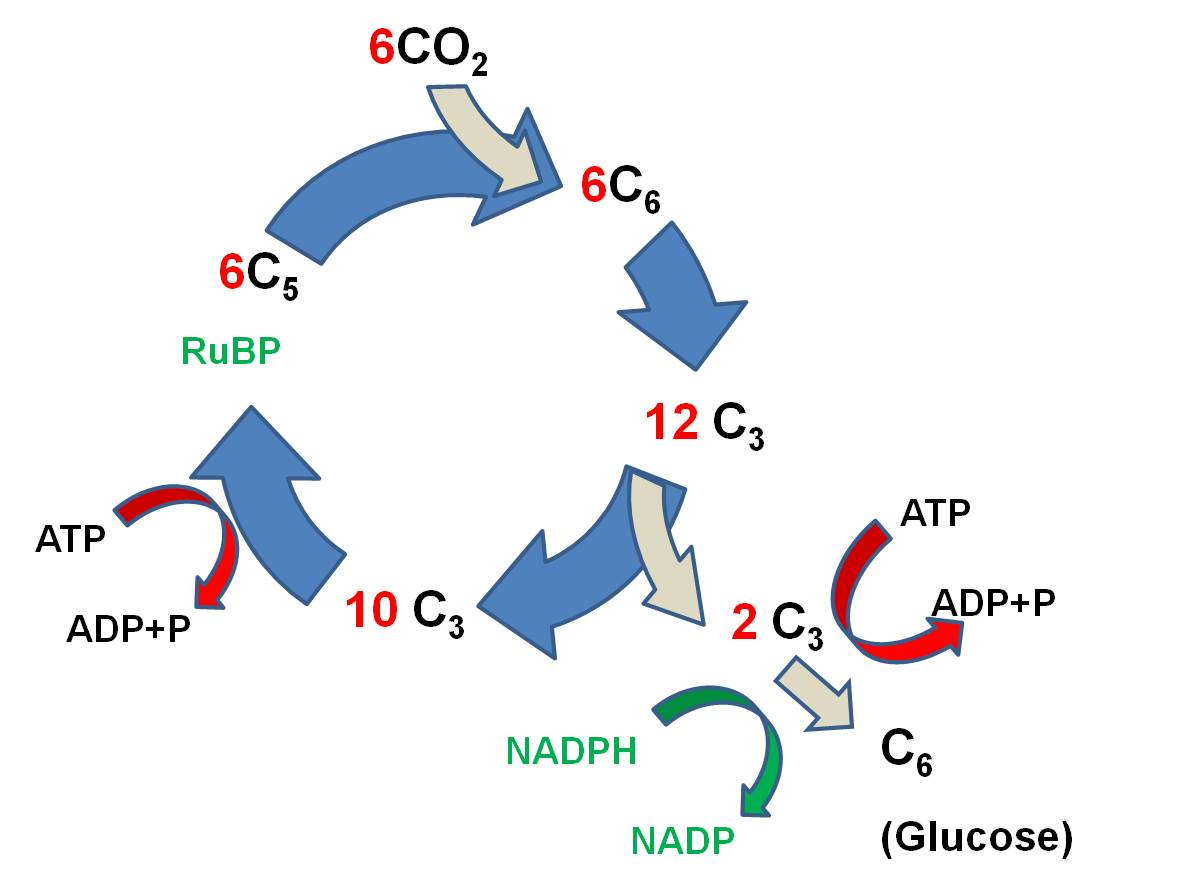 The
primary reaction is called the Calvin-Benson
Cycle, and it works like this:
The
primary reaction is called the Calvin-Benson
Cycle, and it works like this:
- 6 CO2 molecules bind
to 6 C5 molecules of Ribulose Biphosphate (RuBP), making 6 C6 molecules. (ATP is broken and the energy that is released is used to link
CO2 to RUBP).
- These energized C6 molecules
are unstable; the split into 12 C3 molecules. So, since the first
stable product is a C3 molecule, this type of reaction is called the C3 pathway.
- 2 C3 molecules are used
to form 1 glucose (C6) molecule. More ATP is used, and NADPH is used,
too, and H is transferred to put the 'hydrogen' in 'carbohydrate'.
- the 10 remaining C3 molecules (30 C total) are rearranged, using ATP and NADPH, and 6 C5 molecules are generated (30 C total).
The reaction can be summarized like
this: Six CO2 molecules are used to make one molecule of glucose. Six RuBP
molecules are involved, and are recycled through the process. The ATP and NADPH
formed in the light reaction are used to power this reaction; the energy in
these molecules is used top make bonds between the CO2, and the H
from NADPH is used to reduce the CO2 to form glucose (C6H12O6).
As such, the radiant energy initially trapped in chemical bonds in ATP and NADPH
is transferred to form bonds between carbon atoms in glucose. The energy intially
trapped in fragile molecules has been stored in a more stable form.
When cells build glucose from CO2,
they have not only stored energy in a stable form - they have also harvested
carbon from the environment and transformed it into a usable organic molecule.
Since all biologically important molecules (except water) are carbon-based organic
molecules, all life forms needs a source of carbon to build amino acids, nucleotides,
sugars, and lipids. "Heterotrophs" get organic carbon in the 'food'
they eat. "Autotrophs" get their carbon through the light independent
reaction, which also stores energy.
The first group of bacteria discussed
above - the green non-sulphur bacteria and purple non-sulphur bacteria - perform
the Light Dependent Reaction and make ATP using sunlight, but they do not perform
the light indepedent reactions. So, they do not absorb CO2 to make their organic
molecules. Instead, they must consume organic molecules to acquire their carbon.
These organisms are "photoheterotrophs". They may represent the first
step in the evolution of photosynthesis: the evolution of light-trapping reactions
by heterotrophic cells. They use cyclic phosphorylation to make ATP in the presence
of light, but they use organic molecules as electron donors.
Study Questions:
1. Show how the carbons in a glucose (C6) are separated in the steps of respiration.
2. Why is ATP made? Think of the money analogy.
5. What happens
in the electron transport chain? Explain chemiosmosis.
6. How is oxygen
involved in the process of aerobic respiration?
7. Draw what happens
in the primitive light reaction of sulphur bacteria, and explain the events
that occur.
8. What is
the electron donor for sulphur bacteria? What type of limitation does this impose
on where these organisms can live?
9.
Draw and explain what happens in the more advanced light dependent reaction.
Why can we call this an 'adaptation'? (Why is this an improvement over the the
more primitive system, considering the habitats available on Earth?)
10. Draw the Light
Indepedent reaction and describe the events that occur.
11. When, where,
and why is oxygen produced by photosynthesis? What is the primary function
of photosynthesis?
 we will examine the energy harvesting
reactions that ALL living cells perform: Cellular Respiration. In other words, "what happens to the food you eat?" And, "what happens to the oxygen you breather in?" All
living cells - eubacteria, archaea, protists, fungi, plants, and animals - can
harvest the energy contained in the chemical bonds of complex organic molecules.
By breaking the covalent bonds between carbon atoms in these molecules, energy
is released. The energy released by these reactions must be trapped in other bonds or used to do work; otherwise it is lost as heat. So, cells perform coupled reactions to TRAP the energy. One reaction breaks down bonds in food, and the energy released is TRAPPED in new bods formed between
ADP and P --> making ATP. As such, some of the energy
in the covalent bonds of the initial organic molecules is transformed into chemical
energy in bonds of ATP. Carbon-carbon bonds are very strong and stable; enzymes can't break these bonds. These bonds are like a $100 bill--you can't use it everywhere...some stores won't take them. The energy must be converted to a 'lower denominationa; form' that can be used by all enzymes ('stores') in the cell. So, when the 5 carbon-carbon bonds in a glucose are broken, 36-38 bonds are made between ADP and P. And so, each of these bonds in ATP is MUCH weaker (contains less energy)... and most of the energy released by the breaking of the carbon-carbon bonds is lost as heat! Energy in this form is now available to all of the enzymes
in the cell, for catalyzing their own reactions (chemical energy) or doing work
like muscular contraction (mechanical energy) or pumping ions across a membrane
against their concentration gradient (active transport).
we will examine the energy harvesting
reactions that ALL living cells perform: Cellular Respiration. In other words, "what happens to the food you eat?" And, "what happens to the oxygen you breather in?" All
living cells - eubacteria, archaea, protists, fungi, plants, and animals - can
harvest the energy contained in the chemical bonds of complex organic molecules.
By breaking the covalent bonds between carbon atoms in these molecules, energy
is released. The energy released by these reactions must be trapped in other bonds or used to do work; otherwise it is lost as heat. So, cells perform coupled reactions to TRAP the energy. One reaction breaks down bonds in food, and the energy released is TRAPPED in new bods formed between
ADP and P --> making ATP. As such, some of the energy
in the covalent bonds of the initial organic molecules is transformed into chemical
energy in bonds of ATP. Carbon-carbon bonds are very strong and stable; enzymes can't break these bonds. These bonds are like a $100 bill--you can't use it everywhere...some stores won't take them. The energy must be converted to a 'lower denominationa; form' that can be used by all enzymes ('stores') in the cell. So, when the 5 carbon-carbon bonds in a glucose are broken, 36-38 bonds are made between ADP and P. And so, each of these bonds in ATP is MUCH weaker (contains less energy)... and most of the energy released by the breaking of the carbon-carbon bonds is lost as heat! Energy in this form is now available to all of the enzymes
in the cell, for catalyzing their own reactions (chemical energy) or doing work
like muscular contraction (mechanical energy) or pumping ions across a membrane
against their concentration gradient (active transport).  The
"splitting of glucose" (glyco-lysis) is probably an ancient metabolic
reaction; it is performed in the cytoplasm of ALL living cells from prokaryotes
to eukaryotes, and cells can perform this reaction in the presence OR absence
of oxygen gas. So, it seems likely that this was an important energy harvesting
reaction for ancient cells that lived before ~2 bya - before oxygen became abundant
in the oceans and atmosphere. As you can see in the flowchart, glycolysis is
not ONE reaction - it is a series of reactions catalyzed by a variety of enzymes.
For our purposes here, we will consider the primary "inputs" and "outputs"
of the entire reaction, rather than concerning ourselves with each step.
The
"splitting of glucose" (glyco-lysis) is probably an ancient metabolic
reaction; it is performed in the cytoplasm of ALL living cells from prokaryotes
to eukaryotes, and cells can perform this reaction in the presence OR absence
of oxygen gas. So, it seems likely that this was an important energy harvesting
reaction for ancient cells that lived before ~2 bya - before oxygen became abundant
in the oceans and atmosphere. As you can see in the flowchart, glycolysis is
not ONE reaction - it is a series of reactions catalyzed by a variety of enzymes.
For our purposes here, we will consider the primary "inputs" and "outputs"
of the entire reaction, rather than concerning ourselves with each step. b.
Mitochondrial Structure:
b.
Mitochondrial Structure:
 1. 'Gateway' Step:
1. 'Gateway' Step:


 Although all organisms can harvest energy by breaking down organic molecules (Cellular Respiration), some have evolved a mechanism for transforming radiant energy in chemical bond energy. Photosynthesis
is that process of energy transformation. Again, although energy can neither be
created nor destroyed, it can be transformed. In the "Light Dependent Reaction"
radiant energy ('carried' by photons in light) is transformed into chemical
energy ('carried' by electrons). It requires an electron DONOR to provide electrons
that will 'carry' this energy. The energy 'carried' by this electron is used
to form a bond between ADP and P, creating ATP. Through this transfer, the electron
loses this energy. As we have discussed before, the phosphate bonds in ATP are
easily made and easily broken - that's why energy in this form of chemical bond
can be 'used' by all enzymes in the cell. However, ATP is readily hydrolyzed
in water...so it is difficult for a cell to build up a large amount of ATP before
it 'dissolves' to ADP and P again. To store large amounts of energy for a longer
time, the energy in ATP can be converted to a more stable molecule. In most
photosynthetic organisms, the catabolism of ATP is coupled to anabolic reactions
that bind carbon dioxide molecules together into stable molecules of glucose,
for longer term E storage. This also provides the cell with organic carbon that
it can use to make the other biologically important molecules. These are the
"Light Independent Reactions" of photosynthesis.
Although all organisms can harvest energy by breaking down organic molecules (Cellular Respiration), some have evolved a mechanism for transforming radiant energy in chemical bond energy. Photosynthesis
is that process of energy transformation. Again, although energy can neither be
created nor destroyed, it can be transformed. In the "Light Dependent Reaction"
radiant energy ('carried' by photons in light) is transformed into chemical
energy ('carried' by electrons). It requires an electron DONOR to provide electrons
that will 'carry' this energy. The energy 'carried' by this electron is used
to form a bond between ADP and P, creating ATP. Through this transfer, the electron
loses this energy. As we have discussed before, the phosphate bonds in ATP are
easily made and easily broken - that's why energy in this form of chemical bond
can be 'used' by all enzymes in the cell. However, ATP is readily hydrolyzed
in water...so it is difficult for a cell to build up a large amount of ATP before
it 'dissolves' to ADP and P again. To store large amounts of energy for a longer
time, the energy in ATP can be converted to a more stable molecule. In most
photosynthetic organisms, the catabolism of ATP is coupled to anabolic reactions
that bind carbon dioxide molecules together into stable molecules of glucose,
for longer term E storage. This also provides the cell with organic carbon that
it can use to make the other biologically important molecules. These are the
"Light Independent Reactions" of photosynthesis. When
we think of photosynthesis, most of us think "plants". This is generally
correct, but very incomplete. First, there are some plants like Indian Pipe
(Monotropa uniflora) that do not photosynthesize. Although they evolved
from photosynthetic ancestors, they have adopted a parasitic lifestyle and no
longer harvest their own energy from sunlight. In addition, there are photosynthetic
protists (algae and Euglenozoans), and photosynthetic archaeans and eubacteria.
In fact, there are several animals that harbor photosynthetic symbionts, too.
Many corals (corals are animals) ingest algal cells and distribute them to their
tentacles. The algae photosynthesize, and excess sugars are passed to the coral
animal. These symbiotic algae give corals their spectacular colors. When stressed
by water polution or high water temperatures, the corals release their symbionts
and lose their color ("a phenomenon called "coral bleaching").
Long periods without their symbionts results in coral death.
When
we think of photosynthesis, most of us think "plants". This is generally
correct, but very incomplete. First, there are some plants like Indian Pipe
(Monotropa uniflora) that do not photosynthesize. Although they evolved
from photosynthetic ancestors, they have adopted a parasitic lifestyle and no
longer harvest their own energy from sunlight. In addition, there are photosynthetic
protists (algae and Euglenozoans), and photosynthetic archaeans and eubacteria.
In fact, there are several animals that harbor photosynthetic symbionts, too.
Many corals (corals are animals) ingest algal cells and distribute them to their
tentacles. The algae photosynthesize, and excess sugars are passed to the coral
animal. These symbiotic algae give corals their spectacular colors. When stressed
by water polution or high water temperatures, the corals release their symbionts
and lose their color ("a phenomenon called "coral bleaching").
Long periods without their symbionts results in coral death.  Photosynthesis
in prokaryotes occurs on the double-membrane system of these organisms. In eukaryotes,
photosynthesis occurs in organelles called chloroplasts. Chloroplasts have a
bacteria-like double membrane, and they have their own DNA. This DNA is more
similar in most respects to the DNA in free-living bacteria than to the DNA
in the nucleus of the eukaryotic cells they 'inhabit'. For these reasons, most
scientists accept the 'endosymbiotic theory' of chloroplast origins. This theory
states that chloroplasts in the cells of photosynthetic eukaryotes are descendants
of free-living photosynthetic bacteria. At some point in the early evolution
of protists, these photosynthetic bacteria were engulfed by not digested. Rather,
the host cells fed on the excess sugars produced by the internalized bacteria.
Eventually, as the result of gene exchange between the host and proto-chloroplasts,
the eukaryotic host and the prokaryotic symbiont became dependent on one another.
But chloroplasts can still live outside of cells for several days. Plants, evolving
from green algae ancestors, inherited these bacteria-like chloroplasts, too.
Photosynthesis
in prokaryotes occurs on the double-membrane system of these organisms. In eukaryotes,
photosynthesis occurs in organelles called chloroplasts. Chloroplasts have a
bacteria-like double membrane, and they have their own DNA. This DNA is more
similar in most respects to the DNA in free-living bacteria than to the DNA
in the nucleus of the eukaryotic cells they 'inhabit'. For these reasons, most
scientists accept the 'endosymbiotic theory' of chloroplast origins. This theory
states that chloroplasts in the cells of photosynthetic eukaryotes are descendants
of free-living photosynthetic bacteria. At some point in the early evolution
of protists, these photosynthetic bacteria were engulfed by not digested. Rather,
the host cells fed on the excess sugars produced by the internalized bacteria.
Eventually, as the result of gene exchange between the host and proto-chloroplasts,
the eukaryotic host and the prokaryotic symbiont became dependent on one another.
But chloroplasts can still live outside of cells for several days. Plants, evolving
from green algae ancestors, inherited these bacteria-like chloroplasts, too. Photosynthesis
is a critically important process in the evolution and diversity of life. Prior
to the evolution of photosynthesis, life was dependent on absorbing spontaneously
generated organic molecules, or preying on other cells. Neither of these sources
of energy was probably all that common and easy to find. Evolving the ability
to use sunlight as an energy source, which IS abundant and IS easy to find,
meant that life could grow, prosper, and radiate dramatically - almost anywhere
there was a light source. Indeed, it looks like photosynthesis evolved very
early in the history of life; the earliest fossils (stromatolites and filamentous
microfossils dating to ~3.5 by) look very similar to photosynthetic bacteria
that are alive today. When photosynthetic organisms became abundant, they provided
a food supply for a wider variety of heterotrophic cells. Heterotrophs could
then live anywhere phototrophs lived; they were not limited to those rare places
where biological molecules were forming spontaneously. So, complex bacterial
food webs evolved. These early photosynthetic organisms used a primitive form
of photosynthesis that did not produce oxygen as a waste product. So, even though
they flourished for a billion years, no oxygen was added to the atmosphere.
About 2.0 billion years ago, a 'modern' type of photosynthesis evolved that
used water as the electron donor and produced oxygen gas as a waste product.
The production of oxygen gas transformed the oceans (precipitating iron), and
eventually changed the atmosphere, as well. Although oxygen was probably a highly
toxic gas at first (because it is so reactive), life eventually evolved to tolerate
it and then to USE it in oxidative respiration. The evolution of aerobic respiration
allowed for more energy to be harvested from the catabolism of complex organic
molecules, and may have allowed for the evolution of more energy-demanding eukaryotes
and multicellular organisms. As you know, almost all food webs are ultimately
dependent on the photosynthetic organisms at the base of the "food chain"
(hydrothermal vent communities are a possible exception). We use this energy
to stick amino acids together to make our proteins, etc. Even the gas and oil
that powers our industrial societies was initally stored as glucose produced
by photosynthesis. Coal, gas, and oil are just fossilized plants - and we "burn"
that energy millions of years after it was converted from sunlight. We are powering
our societies with sunlight that hit the Earth millions of years ago. But not
only are you (and every other heterotroph) energetically dependant on photosynthetic
organisms for food, you are also indebted to them for changing the planet and
stimulating the evolution of eukaryotic and multicellular life. In short, there
are few processes more important to the history and current function of living
systems (and our petroleum-based economy) than photosynthesis.
Photosynthesis
is a critically important process in the evolution and diversity of life. Prior
to the evolution of photosynthesis, life was dependent on absorbing spontaneously
generated organic molecules, or preying on other cells. Neither of these sources
of energy was probably all that common and easy to find. Evolving the ability
to use sunlight as an energy source, which IS abundant and IS easy to find,
meant that life could grow, prosper, and radiate dramatically - almost anywhere
there was a light source. Indeed, it looks like photosynthesis evolved very
early in the history of life; the earliest fossils (stromatolites and filamentous
microfossils dating to ~3.5 by) look very similar to photosynthetic bacteria
that are alive today. When photosynthetic organisms became abundant, they provided
a food supply for a wider variety of heterotrophic cells. Heterotrophs could
then live anywhere phototrophs lived; they were not limited to those rare places
where biological molecules were forming spontaneously. So, complex bacterial
food webs evolved. These early photosynthetic organisms used a primitive form
of photosynthesis that did not produce oxygen as a waste product. So, even though
they flourished for a billion years, no oxygen was added to the atmosphere.
About 2.0 billion years ago, a 'modern' type of photosynthesis evolved that
used water as the electron donor and produced oxygen gas as a waste product.
The production of oxygen gas transformed the oceans (precipitating iron), and
eventually changed the atmosphere, as well. Although oxygen was probably a highly
toxic gas at first (because it is so reactive), life eventually evolved to tolerate
it and then to USE it in oxidative respiration. The evolution of aerobic respiration
allowed for more energy to be harvested from the catabolism of complex organic
molecules, and may have allowed for the evolution of more energy-demanding eukaryotes
and multicellular organisms. As you know, almost all food webs are ultimately
dependent on the photosynthetic organisms at the base of the "food chain"
(hydrothermal vent communities are a possible exception). We use this energy
to stick amino acids together to make our proteins, etc. Even the gas and oil
that powers our industrial societies was initally stored as glucose produced
by photosynthesis. Coal, gas, and oil are just fossilized plants - and we "burn"
that energy millions of years after it was converted from sunlight. We are powering
our societies with sunlight that hit the Earth millions of years ago. But not
only are you (and every other heterotroph) energetically dependant on photosynthetic
organisms for food, you are also indebted to them for changing the planet and
stimulating the evolution of eukaryotic and multicellular life. In short, there
are few processes more important to the history and current function of living
systems (and our petroleum-based economy) than photosynthesis. a.
cyclic phosphorylation in "purple non-sulphur" and "green non-sulpher"
bacteria:Like all
bacteria, they have a double membrane (two bilayers). Proteins nested within
the inner membrane form "reaction centers (also called "photosystems")
and "electron transport chains" (ETC's) used in photosynthesis. This
inner membrane is often highly convoluted, increasing the surface area and the
number of reaction centers and ETC's that can be imbedded. Each reaction
center contains proteins arrayed around molecules of bacteriochlorophyll, which
contain atoms of Magnesium. In the
presence of light, the photons transfer energy to these electrons. The electrons
are raised to a higher energy state, lost from
the atom, and transferred to an 'electron acceptor molecule' in the inner membrane
of the bacterium, which transfers the electron the the electron transport chain. When a high-energy electron
is transferred down the chain, protons
(H+) follow ('electrostatically') and are pumped across the inner membrane into
the intramembrane space. This build-up of H+ ions in the intermembrane space
creates an electrostatic charge differential across the membrane. There are
closed protein channels that, when opened, allow the H+ to flood through in
response to the charge gradient. This electric discharge energy is used by the
enzyme ATP-synthetase to add a phosphate group to ADP, making ATP. This is called
'chemiosmotic synthesis' or 'chemiosmosis'. So, what has happened is that the
passage of an electron -excited by light energy - has been used to 'pump protons'
into the intermembrane space, establishing an H+ ion charge gradient. The flow
of H+ ions through protein channels transforms this electric energy to chemical
bond energy in the form of a bond between ADP and P--> ATP. The
high-energy electron is then passed down the electron transport chain. and ATP
is produced. The electron, having lost its energy, can be recycled back to the
Mg atom. This cyclic production of ATP, powered by sunlight, is called cyclic
phosphorylation. As discussed below, these odd bacteria do not perform the light
independent pathways. In other words, they do not use the energy in ATP to make
glucose. This has two interesting consequences. First, it means they can't rely
on photosynthesis, alone, for energy harvest, because ATP isn't stable enough
to last over the course of an evening. So, they must also 'eat' - they are heterotrophs,
and can harvest energy from the food they ingest. The other consequence is discussed
below.
a.
cyclic phosphorylation in "purple non-sulphur" and "green non-sulpher"
bacteria:Like all
bacteria, they have a double membrane (two bilayers). Proteins nested within
the inner membrane form "reaction centers (also called "photosystems")
and "electron transport chains" (ETC's) used in photosynthesis. This
inner membrane is often highly convoluted, increasing the surface area and the
number of reaction centers and ETC's that can be imbedded. Each reaction
center contains proteins arrayed around molecules of bacteriochlorophyll, which
contain atoms of Magnesium. In the
presence of light, the photons transfer energy to these electrons. The electrons
are raised to a higher energy state, lost from
the atom, and transferred to an 'electron acceptor molecule' in the inner membrane
of the bacterium, which transfers the electron the the electron transport chain. When a high-energy electron
is transferred down the chain, protons
(H+) follow ('electrostatically') and are pumped across the inner membrane into
the intramembrane space. This build-up of H+ ions in the intermembrane space
creates an electrostatic charge differential across the membrane. There are
closed protein channels that, when opened, allow the H+ to flood through in
response to the charge gradient. This electric discharge energy is used by the
enzyme ATP-synthetase to add a phosphate group to ADP, making ATP. This is called
'chemiosmotic synthesis' or 'chemiosmosis'. So, what has happened is that the
passage of an electron -excited by light energy - has been used to 'pump protons'
into the intermembrane space, establishing an H+ ion charge gradient. The flow
of H+ ions through protein channels transforms this electric energy to chemical
bond energy in the form of a bond between ADP and P--> ATP. The
high-energy electron is then passed down the electron transport chain. and ATP
is produced. The electron, having lost its energy, can be recycled back to the
Mg atom. This cyclic production of ATP, powered by sunlight, is called cyclic
phosphorylation. As discussed below, these odd bacteria do not perform the light
independent pathways. In other words, they do not use the energy in ATP to make
glucose. This has two interesting consequences. First, it means they can't rely
on photosynthesis, alone, for energy harvest, because ATP isn't stable enough
to last over the course of an evening. So, they must also 'eat' - they are heterotrophs,
and can harvest energy from the food they ingest. The other consequence is discussed
below.
 b.
"green sulphur" and "purple
sulphur" bacteria that
use sulphides as the electron donors:
b.
"green sulphur" and "purple
sulphur" bacteria that
use sulphides as the electron donors: - Here's how it works: Light strikes the phosystems nested in the inner membrane
(called the 'thylakoid' membrane in chloroplasts). An electron in each photosystem
is excited and lost from the Mg in the chlorophyll molecule. The electrons are
accepted by partcular electron acceptor molecules. The electron lost from PS
I is ultimately passed to NADP, which accepts a H+ to balance the charge, making
the high energy molecule, NADPH. The electron lost from PSII is passed to an
electron acceptor, and then to molecules in the electron transport chain. As
the electron is passed down the chain, ATP is produced by chemiosmosis (as described
above). When this electron has lost it's energy, it replaces the electron lost
from PS I. So, PS I is all set, and need not strip electrons from an electron
donor. However, PS II has lost an electron, and must replace this electron for
photosynthesis to continue. PSII strips electrons from H2O. Water
is split into oxygen, 2 H+, and 2 electrons. The electrons are passed to the
cholorophyll in PS II, excited by light, and energized. The oxygen reacts with
another oxygen atom to produce oxygen gas, which is released as a waste product.
The propose of photosynthesis is not "to produce oxygen". The purpose
of the light reaction of photosynthesis is to transform radiant energy into
chemcial energy, and produce ATP and NADPH. The two molecules, ATP and NADPH,
are the useful products. Again, oxygen gas is produced as a waste product when
electrons are stripped from water. The presence of oxygen in the oceans 2.5-2
billion years ago, indicated by the presence of sedimentary deposits with oxidized
iron (banded iron formations), indicates the evolution of this more advanced
type of photosynthesis that evolved in ancient photosynthetic bacteria.
- Here's how it works: Light strikes the phosystems nested in the inner membrane
(called the 'thylakoid' membrane in chloroplasts). An electron in each photosystem
is excited and lost from the Mg in the chlorophyll molecule. The electrons are
accepted by partcular electron acceptor molecules. The electron lost from PS
I is ultimately passed to NADP, which accepts a H+ to balance the charge, making
the high energy molecule, NADPH. The electron lost from PSII is passed to an
electron acceptor, and then to molecules in the electron transport chain. As
the electron is passed down the chain, ATP is produced by chemiosmosis (as described
above). When this electron has lost it's energy, it replaces the electron lost
from PS I. So, PS I is all set, and need not strip electrons from an electron
donor. However, PS II has lost an electron, and must replace this electron for
photosynthesis to continue. PSII strips electrons from H2O. Water
is split into oxygen, 2 H+, and 2 electrons. The electrons are passed to the
cholorophyll in PS II, excited by light, and energized. The oxygen reacts with
another oxygen atom to produce oxygen gas, which is released as a waste product.
The propose of photosynthesis is not "to produce oxygen". The purpose
of the light reaction of photosynthesis is to transform radiant energy into
chemcial energy, and produce ATP and NADPH. The two molecules, ATP and NADPH,
are the useful products. Again, oxygen gas is produced as a waste product when
electrons are stripped from water. The presence of oxygen in the oceans 2.5-2
billion years ago, indicated by the presence of sedimentary deposits with oxidized
iron (banded iron formations), indicates the evolution of this more advanced
type of photosynthesis that evolved in ancient photosynthetic bacteria.
 The
primary reaction is called the Calvin-Benson
Cycle, and it works like this:
The
primary reaction is called the Calvin-Benson
Cycle, and it works like this: