 B. Properties
of Atoms
B. Properties
of Atoms
1. conventional matter - defined
as a substance that has mass and occupies space - consists of atoms.
2. there are 94 different types of naturally occurring atoms - defined by differences
in the number of protons in the nucleus. These are the naturally occurring elements.
Atoms are also defined as the smallest unit of a pure substance (one element)
that retains the properties of that substance and cannot be subdivided further
by chemical means.
3. compounds are substances composed of two or more types of elements in a fixed
ratio, with a particular structure/spatial arrangement maintained by chemical
bonds (ionic or covalent). So, NaCl (table salt) is an ionic compound. H2O (water)
is a covalent compound.
4. molecules are substances
of two or more atoms bound together by covalent bonds. So, it is inappropriate
to refer to a 'molecule' of NaCl (which is ionic). Two atoms of hydrogen bound
together by a covalent bond is a molecule of hydrogen. It is not a compound,
as it only contains one type of element. For compounds consisting of covalently
bound atoms, a molecule is the smallest unit of the compound that maintains
the properties of that compound. So, we can have one molecule of water, which
is also a compound.
 B. Properties
of Atoms
B. Properties
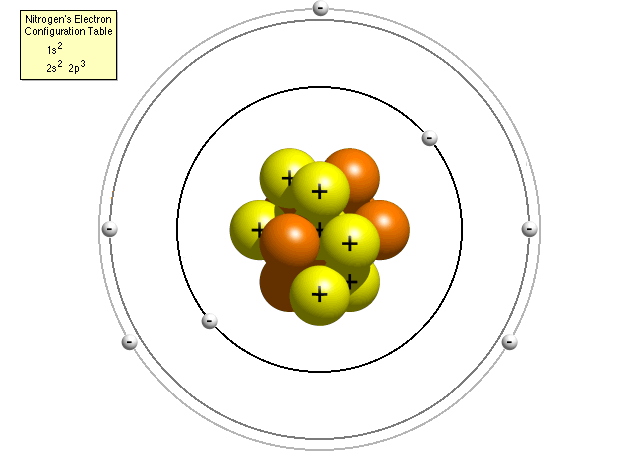
of AtomsWe will use a very simplistic 'Bohr' model of atomic structure, emphasizing the particulate nature of matter (rather than the wave nature) and excluding a consideration of quarks. For a more detailed account, feel free to see this link from the University of Oregon and Wikipedia.
1. atoms have a nucleus containing
protons (mass ~ 1 atomic mass unit; elementary charge = +1 ) and neutrons (mass
~ 1 atomic mass unit; elementary charge = 0). The number of protons defines
the type of atom - the element - and it's atomic number. The mass of an atom
is largely determined by the mass of protons and neutrons. Although all atoms
of an element have the same number of protons, the number of neutrons can vary.
These atoms with variable numbers of neutrons are called isotopes. Those with
an excess number of neutrons are less stable and will loose them over time.
These are radioisotopes, and they emit energy when they loose a neutron. They
loose them at a constant rate, so you can date how old they are by how many
have changed to the more stable state. Atoms with unequal numbers of protons
and electrons have a net charge and are called ions.
2. the nucleus is surrounded by a cloud of electrons (mass ~ 0; elementary charge
= -1), represented as shells and orbitals: Shells 1, 2, 3 have 1, 4, 4orbitals,
'containing' a maximum of 2, 8, 8 electrons, respectively. The orbitals are
1000's of times the width of the nucleus, so an atom (and hence, all matter)
is mostly space. For comparison, if you envision the nucleus of a carbon atom
as a basketball (about 12 inches in diameter), the outermost electrons would
be orbiting 5 miles away.
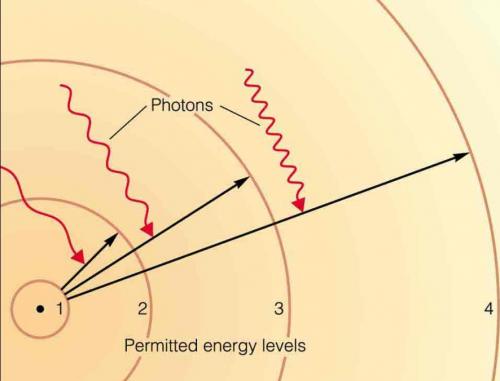 3. the distance
between the orbital and the nucleus correlates with the energy of the electron
(in terms of a wave function).
3. the distance
between the orbital and the nucleus correlates with the energy of the electron
(in terms of a wave function).
4. as electrons gain energy, they 'orbit' farther from nucleus. Without an input
of energy, an atom will lose energy and approach it's lowest energy state, occupying
the closest 'unoccupied' orbital available.
5. changing orbitals - and energy states - is a discrete process. Electrons
must gain or lose discrete amounts of energy (quanta) to change position to
another orbital.
6. Binding properties are largely governed by the number of electrons in the
outermost orbital/shell. They achieve greater stability when the outermost orbitals/shells
are full. They achieve this stability by losing, gaining, or sharing electrons
with other atoms.
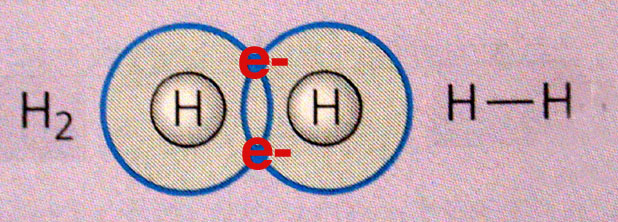 A.
Covalent Bonds
A.
Covalent Bonds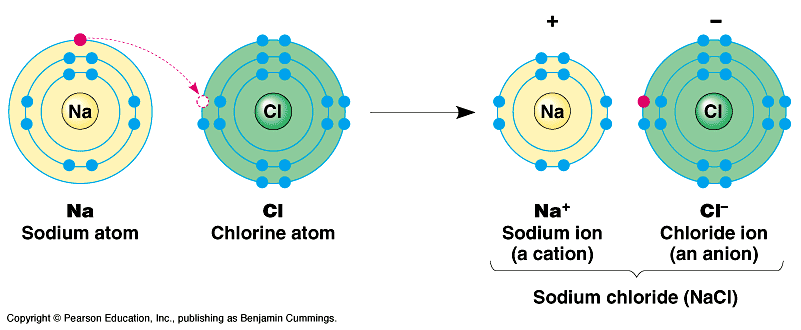 B.
Ions and Ionic Bonds
B.
Ions and Ionic BondsIf the attraction between nuclei is very unequal, the shared electrons can be stripped from one of the atoms and taken by the other. This creates charged particles (ions) which may then be attracted to one another based on their opposite charge. (NaCl)
These are weak ionic bonds, weak forces of attraction between a partially charged Hydrogen Atom (+ charge) and a negatively charged molecule or the negative portion of a molecule.
III. Molecules
 A.
Water
A.
Water
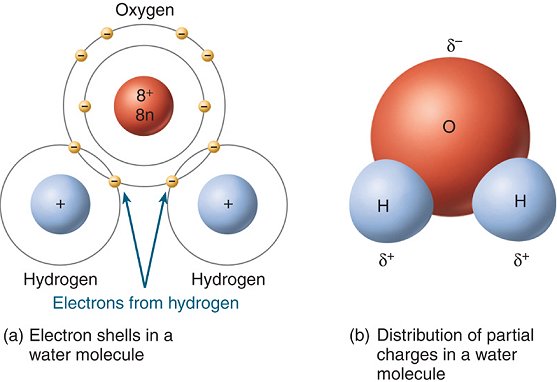
1. Structure
- H2O
is a polar molecule with partially positive hydrogen ends and a partially negative
oxygen end
- thus, water
forms hydrogen bonds with other water molecules, and other charged substances
(polar or ionic).
2. Properties and Their Importance
a. often called a 'universal' solvent because polar and ionic compounds dissolve in water; once dissolved, they can interact with other solutes. By 'dissolving', we mean the separation of molecules or ions from one another. This happens because the water molecules can get between the molecules/ions and bind to them, separating these molecules/ions in solution.
b. water has a 'high specific heat' - meaning that it takes lots of energy to change its temperature and state. So, aqueous solutions (cells and aqueous environments) are thermally stable.
c. water dissociates into H+ and OH- ions, at a concentration of 1 x 10-7 molecules in pure water = pH of 7.
d. water is 'cohesive', in that it 'sticks' to iself; and it is'adhesive', in that it sticks to charged surfaces. Capillary action in vascular plants and small blood vessels is a function of these properties.
 B.
Carbohydrates
B.
Carbohydrates
1. Structure:
- monomer - monosaccharide (simple sugar) - CnH2nOn
(glucose, galactose, fructose are 6 carbon sugars; ribose, ribulose, deoxyribose
are 5 carbon sugars)
- disaccharides: sucrose (glucose + fructose); maltose - (2 glucose)
- polymer - polysaccharide - chain of sugars
starch, glycogen, chitin, cellulose
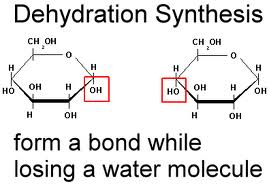
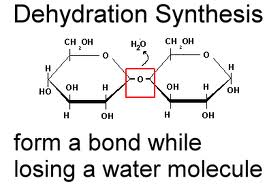 - monomers are linked together into polymers using dehydration synthesis - a
removal of a water molecule (dehydration) and the synthesis of a bond. This
requires energy and is catalyzed by enzymes in living systems.
- monomers are linked together into polymers using dehydration synthesis - a
removal of a water molecule (dehydration) and the synthesis of a bond. This
requires energy and is catalyzed by enzymes in living systems.
2. Function:
a. energy storage:
- all large biomolecules have lots of bonds and thus store lots of energy.
But, the larger the molecule, the more time it takes to harvest all the energy
by metabolic breakdown (catabolism). So, polysaccarides serve better as
'longer-term' energy storage than monosaccharides, whereas monosaccarides, because
they can be metabolized more quickly, serve better as a short term energy supply.
(starch in plants andglycogen in the liver of animals are longer term storage
molecules; glucose is the short-term energy molecule in all of life)
b. Structural:
- cellulose is just a long chain of glucose. And decomposers break down
wood to create the sugars they will use for metabolic energy.
- chitin is the primary component of exoskeletons in arthropods.
 C. Proteins - (we
will cover this in more detil during the lecture on protein synthesis)
C. Proteins - (we
will cover this in more detil during the lecture on protein synthesis)
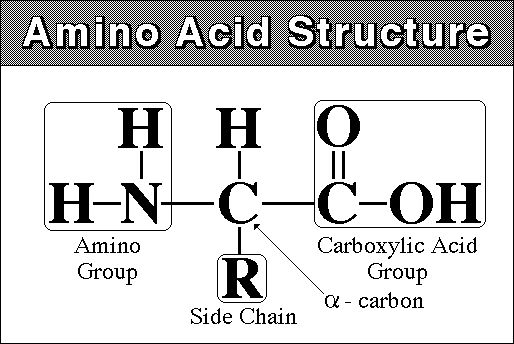
1. Structure:
monomer - amino acid - amine (NH2) group at one end and carboxyl
group (COOH) at other
there are 20 different amino acids that are found in living systems.
polymer - polypeptide - 100 to 300 amino acids long. The AA's are linked by dehydration synthesis reactions into a long linear chain. Because there are 20 Amino Acids ("letters") that can be used in their construction, proteins can have a limitless number of different combinations (like letters in different combinations make differnt "words"). This variety in form means variety in function.
**Higher levels of structure:
1. the primary structure of a polypeptide/protein is the linear sequence of amino acids
2. this linear sequence can take a helical or "pleated" sheet shape, depending on bond angles and soforth. These are secondary levels structure
3. some proteins then fold upon themselves, taking a globular shape. This globular shape is maintained by bonds between different functional groups of differnt amino acids. Enzymes and cell membrane proteins are common globular proteins.this is called tertiary structure.
4. Sometimes, single proteins are not functional on their own - they must be combined with other proteins to forma a protein with a quaternary structure. Hemoglobin, with 2 alpha and 2 beta globular polypeptides, is one example. collagen is another, composed of several helical polypeptides.
2. Function:
a. Energy Storage: (all biomolecules
can be broken down for energy harvest. Typically, since proteins are doing
something else, too, they are broken down last so that the organism can maintain
this function that the protein performs for as long as possible).
b. Structural:
after water, animals are largely proteinaceous
collagen, elastin, muscle proteins, etc.
c. Metabolic:
all biological reactions are catalyzed. Most biological catalysts are
proteinaceous ENZYMES
d. transport:
cell membrane - there are proteins that assist transport across the membrane
organism - hemoglobin, for instance, transport oxygen
e. Immunity:
antibodies are proteins.
1. Structure:
monomer -
fatty acid - long carbon chain with a carboxyl group (COOH)
- can be saturated (with H - no double bonds between C's) or unsaturated (a
double bond)
- animal fats are usually saturated, and are solid at room temp. Plant and fish
fats are usually unsaturated, and are liquid at room temp and are called 'oils'.
By saturating a plant fat, it can be made solid - hydrogenated fat or oil. (changing
peanut oil into peanut butter, or vegetable oil into "crisco"). During
this process, trans-fats are also created. These are unsaturated fats with a
trans (not cis) conformation. Trans-fats have been associated with atherosclerosis
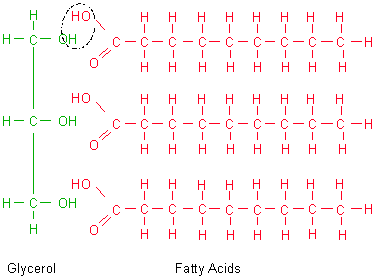 polymer - fat (triglyceride)
polymer - fat (triglyceride)
- three fatty acids attached by dehydration synthesis to a glycerol molecule
- phospholipid: glycerol with 2 fatty acids and a phosphate (PO4) group.
The PO4 is negatively charged (thus, polar), while the fatty acids are non-polar.
This accounts for how these molecules orient in aqueous solutions, forming membranes.
A "choline" groups is typically attached to the phosphate.
2. Function:
a. Energy storage: saturated fats
are very dense - the fatty acids fit together, in parallel, very snugly.
So, to store the most energy in the smallest space, fats are the preferred medium.
b. Cell membranes - barrier to water soluble materials. The non-polar lipid "bilayer" is a barrier to water soluble materials (that are ionic or polar). So, ionic and polar compounds can't just flow into the cell; the cell can regulate how much of what gets in and out.
c. Insulation
d. Hormones - derived from fats,
lipid soluble, slip right theough cell membranes into cells, so they can function
at very low concentrations.
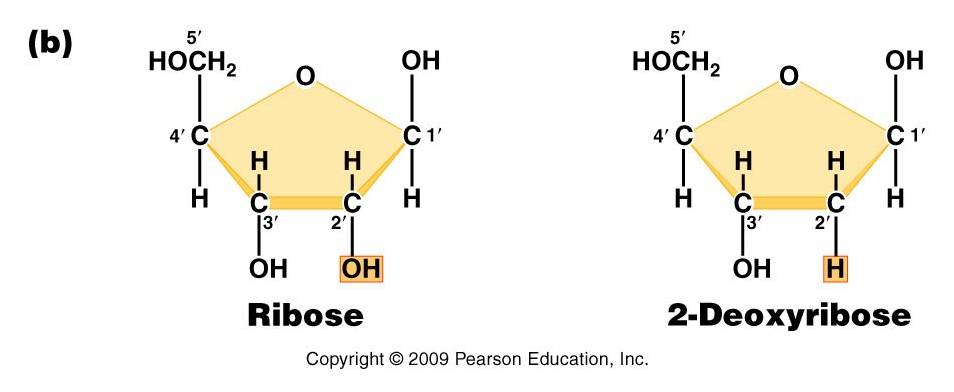
DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) are nucleic acids - polymers consisting of a linear sequence of linked nucleotide monomers. We will describe the structure of the monomers first, and then describe how they are linked into linear polymers. Finally, we will describe the double-stranded structure of ds-DNA.
1. The monomers are "nucleotides"
three components:
- Pentose (5 carbon) sugar: either ribose (RNA) or deoxyribose (DNA). The carbons are numbered clockwise. The difference between the sugars is that ribose has an -OH group on the 2' carbon, whereas deoxyriboes has only 2 H groups and thus is "deoxygenated" relative to ribose. BOTH sugars have an -OH group on the 3' carbon, which will be involved in binding. The 5' carbon is a sidegroup off the ring.
- Nitrogenous Base: each nucleotide has a single nitrogenous base attached to the 1' carbon of the sugar. This nitrogenous base may be a double-ringed structure (purine) or a single ringed (pyrimidine) structure. The purines are adenine (A) and guanine (G). The pyrimidines are thymine (T), cytosine (C), and uracil (U). DNA nucleotides may carry A, G, C, or T. RNA nucleotides carry either A, G, C, or U.
- The third component of a nucleotide is a phosphate group, which is attached to the 5' carbon of the sugar. When a nucleotide is incorporated into a chain, it has a single phosphate group. However, nucleotides can occur that have two or three phosphate groups (dinucleotides and trinucleotides). ADP and ATP are important examples of these types of molecules. In fact, the precursors of incorporated nucleotides are trinucleotides. When two phosphates are cleaved, energy is released that can be used to add the remaining monophosphate nucleotide to the nucleic acid chain.
2. Polymerization is by 'dehydration synthesis'
As with all other classes of biologically important polymers, monomers are linked into polymers by dehydration synthesis. In nucleic acid formation, this involves binding the phosphate group of one nucleotide to the -OH group on the 3' carbon of the existing chain. For the purposes of seeing how this reaction works, we can envision an H+ on one of the negatively charged oxygens of the phosphate group. Then, a molceule of water can be removed from these two -OH groups, leaving an oxygen binding the sugar of one nucleotide to the phosphate of the next.
This creates a 'dinucleotide'. It has a polarity/directionality; it is different at its ends. At one end, the reactive group is the phosophate on the 5' carbon. This is called the 5' end of the chain. At the other end, the reactive group is the free -OH on the 3' carbon; this is the 3' end of the chain. So, a nucleic acid strand has a 5' - 3' polarity.
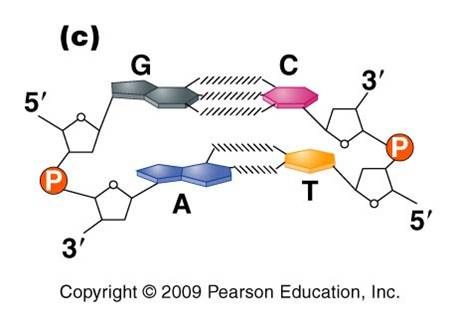 3.
Most DNA exists as a 'double helix' (ds-DNA) containing two linear nucleic acid
chains.
3.
Most DNA exists as a 'double helix' (ds-DNA) containing two linear nucleic acid
chains.
a. the nitrogenous bases on the two strands are 'complementary' to each other, and form weak hydrogen bonds between them. A always pairs with T, and C always pairs with G. As such, there is always a double-ringed purine pairing with a single-ringed pyrimidine, and the width of the double-helix is constant over its entire length.
b. the two strands (helices) are anti-parallel: they are arranged with opposite polarity. One strands points 5' - 3', while the other points 3' - 5'. The direction of the pentose sugars and the type of reactive group at the ends of the chains show this relationship.
4. RNA performs a wide variety of functions in living cells:
a. m-RNA (for "messenger") is the copy of a gene. It is the sequence of nitrogenous bases in m-RNA that is actually read by the ribosome to determine the structure of a protein.
b. r-RNA (for "ribosomal") is made the same way, as a copy of DNA. However, it is not carrying the recipe for a protein; rather, it is functional as RNA. It is placed IN the Ribosome, and it helps to ‘read’ the m-RNA.
c. t-RNA (for "transfer") is also made as a copy of DNA, but it is also functional as an RNA molecule. Its function is to bind to a specific amino acid and incorporate it into the amino acid sequence as instructed by the m-RNA and ribosome.
d. mi-RNA (micro-RNA) and si-RNA (small interfering RNA) bind to m-RNA and splice it; inhibiting the synthesis of its protein. This is a regulatory function.
e. sn-RNA (small nuclear RNA) are short sequences that process initial m-RNA products, and also regulate the production of r-RNA, maintain telomeres, and regulate the action of transcription factors. Regulatory functions.
Study Questions:
1) What is the basic structure of an atom (mass, charge, and location of its components)?
2) What is the relationship between electrons, orbital distance, and energy?
3) What are isotopes and ions?
4) What are covalent, ionic, and hydrogen bonds?
5) Draw the structure of water, including all electrons in their shells. Also identify the covalent bonds, and the position of the partial charges on the molecule.
6) What two large classes of compounds dissolve in water? Why?
7) What is the basic structure of a monosaccharide?
8) What type of molecule are chitin and cellulose?
9) Show how two monosaccharides are linked together. What is the name of this reaction?
10) What is the structure of a fatty acid? A triglyceride?
11) What is the monomeric unit of proteins? Draw one, without specifying the variable group.
12) Show how two amino acids are linked together. What is the name of the reaction?
13) Diagram the parts of an RNA nucleotide.
14) Show how two nucleotides are linked together by dehydration synthesis reactions.
15) Why does the purine - pyrimidine structure relate to the complementary nature of double-stranded DNA?
16) Draw a DNA double helix, showing three base pairs and the antiparallel nature of the helices.
17) Describe the higher levels of eukaryotic chromosome structure, including the terms nucleosome and solenoid.